Esterase mutant and application thereof
A technology of mutants and esterases, which is applied in the field of preparation of optically active 2-aryl propionic acid, can solve the problems of poor stereoselectivity of ketoprofen, and achieve reduced production costs, high enzyme activity and/or stereoselectivity Sexuality, the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Codon optimization of Est-AF
[0031] According to the codon usage frequency distribution table of E. coli, all amino acids in the esterase Est-AF sequence SEQ ID NO: 1 were translated using the optimal codons in the codon usage frequency distribution table. In addition, it is necessary to avoid repetitive sequences and avoid NcoI and HindIII restriction endonuclease sites generated during codon optimization. Finally, the optimized sequence SEQ ID NO:2 was obtained.
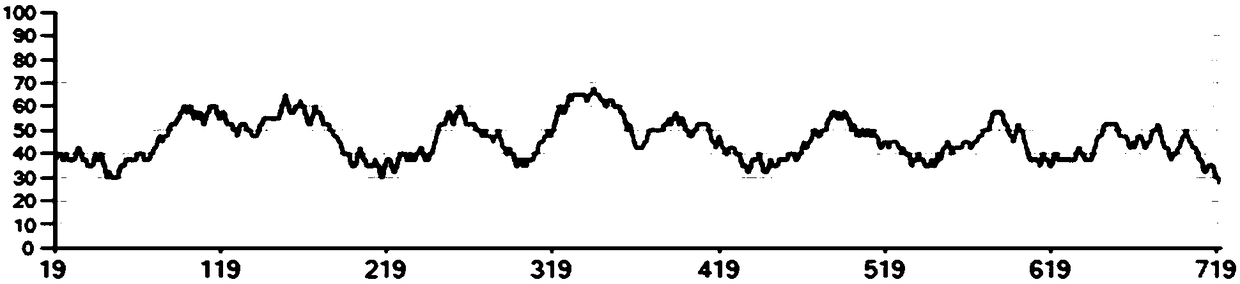
[0032] The optimized sequence GC content distribution is as follows figure 1 shown. Finally, NcoI and HindIII restriction sites were added to the two ends of the sequence, which was synthesized by General Biosystems (Anhui) Co., Ltd., and the synthesized sequence was named Est1.
Embodiment 2
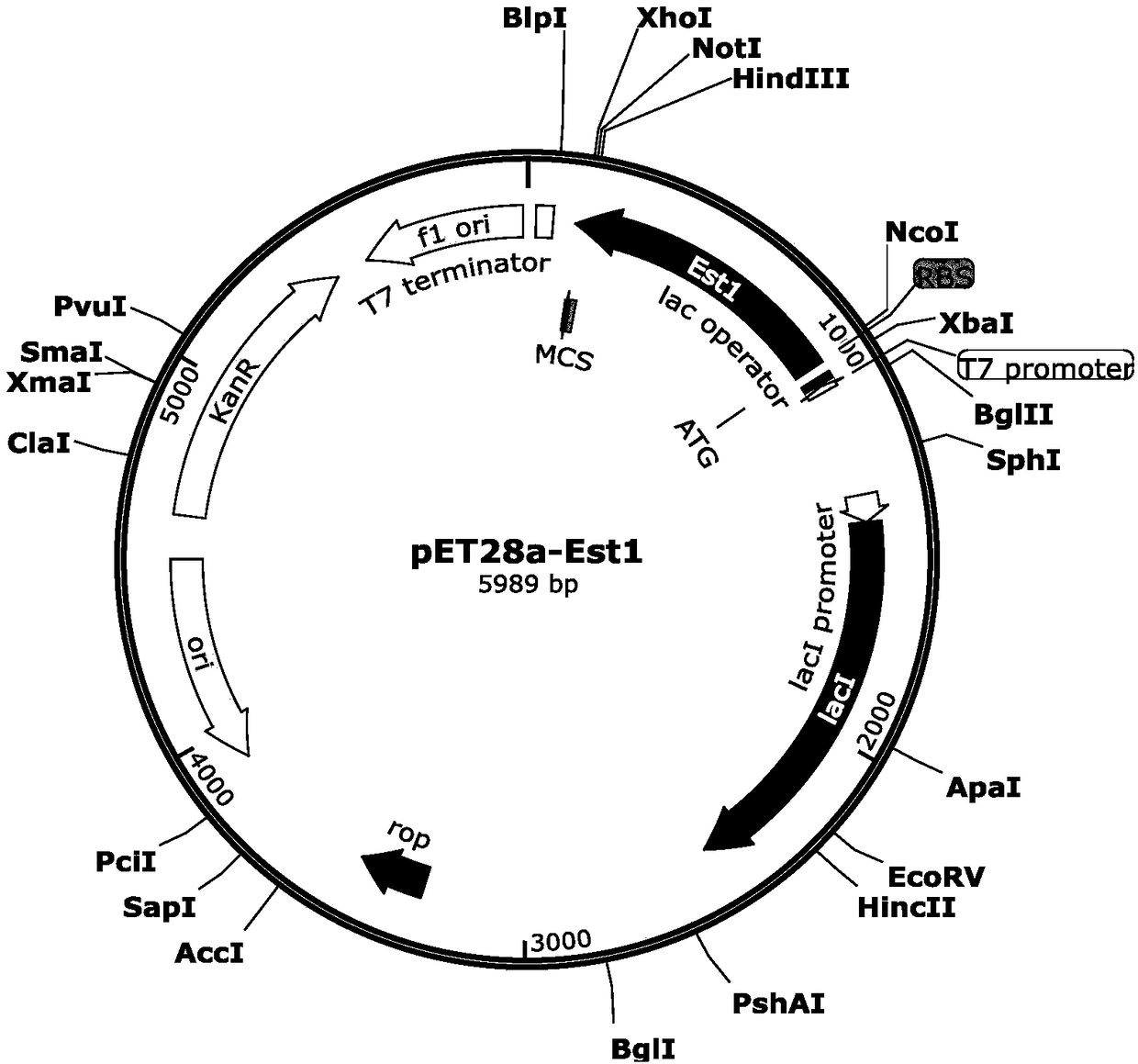
[0033] Example 2 Construction of Est1 expression strain
[0034] Using the synthesized Est1 sequence as a template, the following primers were used to amplify the Est1 gene fragment with a length of about 740 bp.
[0035] 5'-CC CCATGG AACGTATTACCCTG-3' (SEQ ID NO: 3)
[0036] 5'-CC AAGCTT TTACAGTTTTTCAATAAA-3' (SEQ ID NO: 4)
[0037] PCR system: 5×PrimeSTAR PCR HS Buffer 10μL, upstream primer and downstream primer 1μL (10pM), DNA template 1μL (0.1μg), dNTPs (2.5mM) 4μL, PrimeSTAR PCR HS Polymerase 0.5μL and ddH 2 0 to 50 μL.
[0038] PCR amplification steps are: (1) Pre-denaturation at 98°C for 20s; (2) Denaturation at 98°C for 10s; (3) Annealing at 60°C for 10s; (4) Extension at 72°C for 60s; (2)~(4) repeat 30s (5) Continue to extend for 10 min at 72°C.
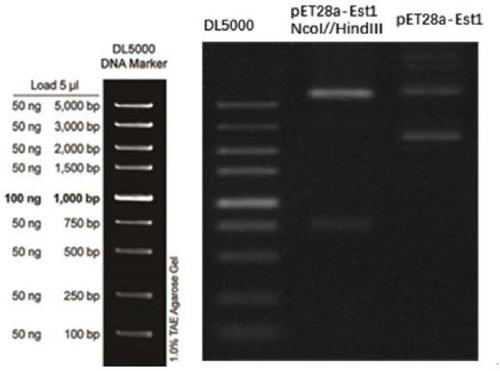
[0039] The PCR product was purified by agarose gel electrophoresis, and the target band of about 1100 bp was recovered using an agarose gel DNA recovery kit.
[0040] The PCR product was double-digested with restric...
Embodiment 3
[0042] Expression of embodiment 3 recombinant esterases
[0043]The recombinant Escherichia coli E. coli BL21(DE3) / pET28a-Est1 obtained in Example 2 was inoculated into LB medium containing kanamycin (50 mg / L), and cultured overnight at 37° C. with shaking. According to the inoculum amount of 2‰ (v / v), insert into the 250ml Erlenmeyer flask that 50ml LB culture medium is housed, put 37 ℃, 200rpm shaker shaking culture. When the OD600 of the culture medium reached 0.8, IPTG with a final concentration of 0.5 mmol / L was added for induction. After induction at 30°C for 8 hours, the culture medium was centrifuged to collect the cells to obtain about 0.3 g of wet cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com