Modifying method for heterocycle aramid and modified heterocycle aramid
A heterocyclic aramid fiber and modified technology, applied in the field of polymer materials, can solve the problems of destroying the PPTA aggregated state structure and decreasing the modulus, achieving simple and convenient operation, improved compressive strength and interlaminar shear strength, and improved extreme sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a modified heterocyclic aramid fiber, the preparation method of which comprises:
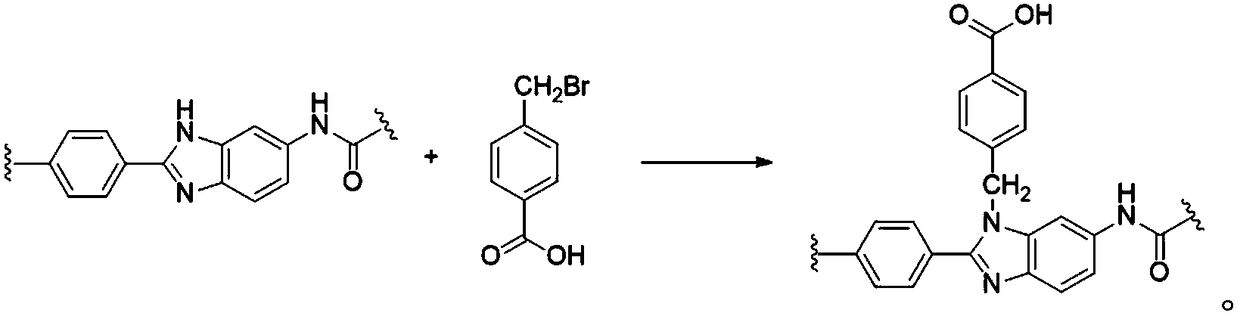
[0040] S1. Under an inert atmosphere, add 0.5g of BBA to 100ml of DMAc and 100ml of acetonitrile respectively, and stir until the BBA is dissolved. Then, 0.5 g of heterocyclic aramid fiber (monomer molar ratio TPC:PABZ:PDA=10:5:5) was added to the DMAc / BBA solution to swell for 30 minutes. Then statically heat-treat at 320° C. for 30 minutes to obtain a cross-linked heterocyclic aramid fiber.
[0041] S2. The above-mentioned cross-linked heterocyclic aramid fiber was swelled in an acetonitrile / BBA solution for 30 minutes, and then heat-treated at 230° C. for 30 minutes to obtain a modified heterocyclic aramid fiber.
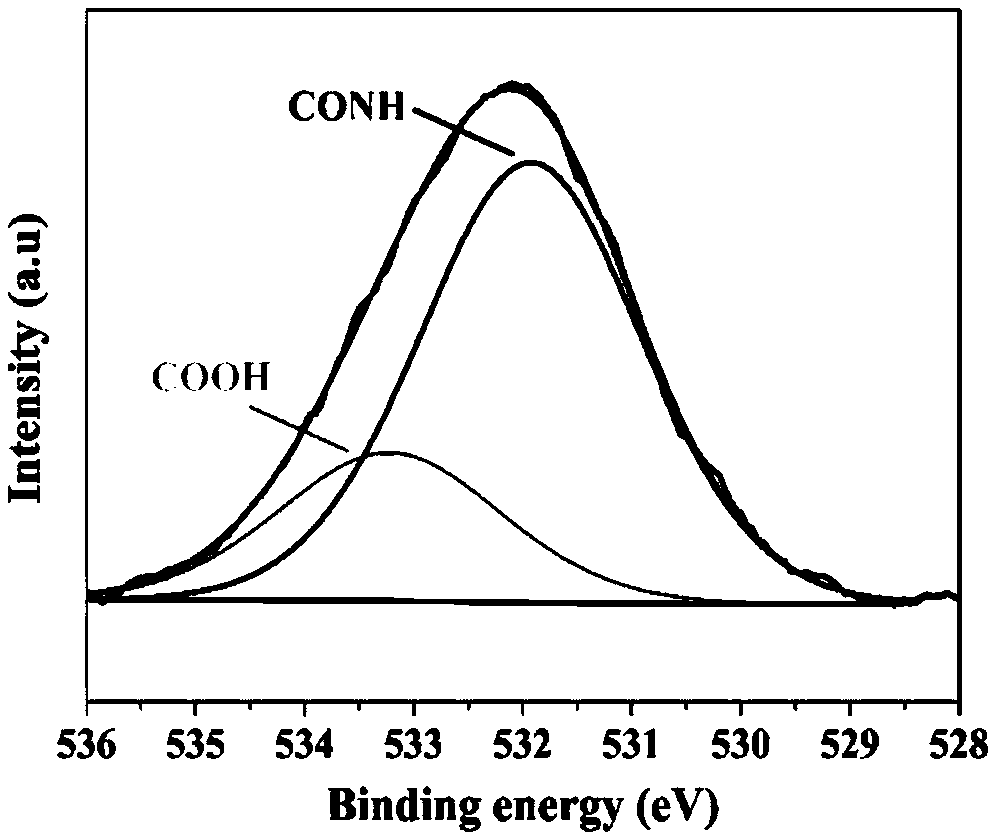
[0042] The XPS spectrum of the modified heterocyclic aramid fiber provided by the present embodiment is as follows: figure 1 As shown, the peak of the amide bond and the peak of the carboxyl group can be seen from the XPS spectrum, indicating that ...
Embodiment 2
[0044] This embodiment provides a modified heterocyclic aramid fiber, the preparation method of which comprises:
[0045] S1. Under an inert atmosphere, add 0.3g of BBA to 100ml of DMAc and 100ml of acetonitrile respectively, and stir until the BBA is dissolved. Then 0.5 g of heterocyclic aramid (TPC:PABZ:PDA=10:7:3) was added to the DMAc / BBA solution to swell for 45 minutes. Then statically heat-treat at 320° C. for 30 minutes to obtain a cross-linked heterocyclic aramid fiber.
[0046] S2. Swell the above-mentioned cross-linked heterocyclic aramid fiber in the acetonitrile / BBA solution for 30 minutes. Then heat treatment at 230° C. for 0.5 h to obtain a modified heterocyclic aramid fiber.
Embodiment 3
[0048] This embodiment provides a modified heterocyclic aramid fiber, the preparation method of which comprises:
[0049] Under an inert atmosphere, 0.4g of BBA was added to 100ml of NMP and 100ml of acetonitrile respectively, and stirred until the BBA was dissolved. Then, 0.5 g of heterocyclic aramid (TPC:PABZ:PDA=10:6:4) was added to the NMP / BBA solution to swell for 30 minutes. Then statically heat-treat at 310° C. for 30 minutes to obtain a cross-linked heterocyclic aramid fiber.
[0050] S2. Swell the above-mentioned cross-linked heterocyclic aramid fiber in the acetonitrile / BBA solution for 40 minutes. Then heat treatment at 250° C. for 15 minutes to obtain a modified heterocyclic aramid fiber.
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com