Genetically modified non-human mammals by multi-cycle electroporation of cas9 protein
A non-human mammal, genetic modification technology, applied in the field of genetically modified non-human mammals produced by multi-cycle electroporation of CAS9 protein, can solve the problem of unsuccessful germline transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256] Example 1 Electroporation of mouse zygotes and putative zygotes with reporter-encoding polynucleotides
[0257] This experiment describes the results of electroporation of mouse zygotes or putative zygotes ("zygotes" for short) with a reporter-gene-encoding plasmid.
[0258] The pMAXGFP vector (Lonza, USA) carrying the CMV promoter and SV40 polyadenylation signal supporting ubiquitous expression was chosen for this experiment. B6D2F2 mouse embryos were collected and treated in acidic Tyrode solution (AT) (P / N T1788, SigmaAldrich) for 5 s, washed twice in KOSMaa / BSA (P / N Zeks-050, Zenith Biotech), and placed in 25 μL of Opti-MEM (P / N 31985, Life Technologies). Embryos in 25 μL were then mixed with an equal volume (25 μL) of 80 ng / μL of pMAXGFP in TE buffer (10 mM Tris, 0.1 mM EDTA, pH 7.5) to achieve a final DNA concentration of 40 ng / μL, and the mixture was Load into a 1-mm shock cuvette and perform electroporation using the following settings: 30 V, pulse duration 1 ...
Embodiment 2
[0262] Example 2 Electroporation of mouse zygotes and putative zygotes with CRISPR / Cas system
[0263] This experiment demonstrates the use of the methods of the present invention to deliver the CRISPR / Cas system to mouse zygotes or putative zygotes ("zygotes" for short) for CRISPR / Cas-mediated targeted gene disruption.
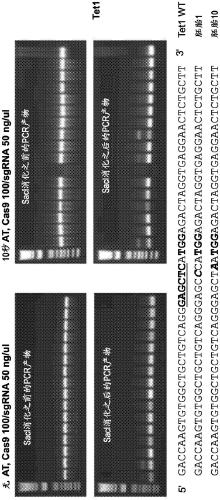
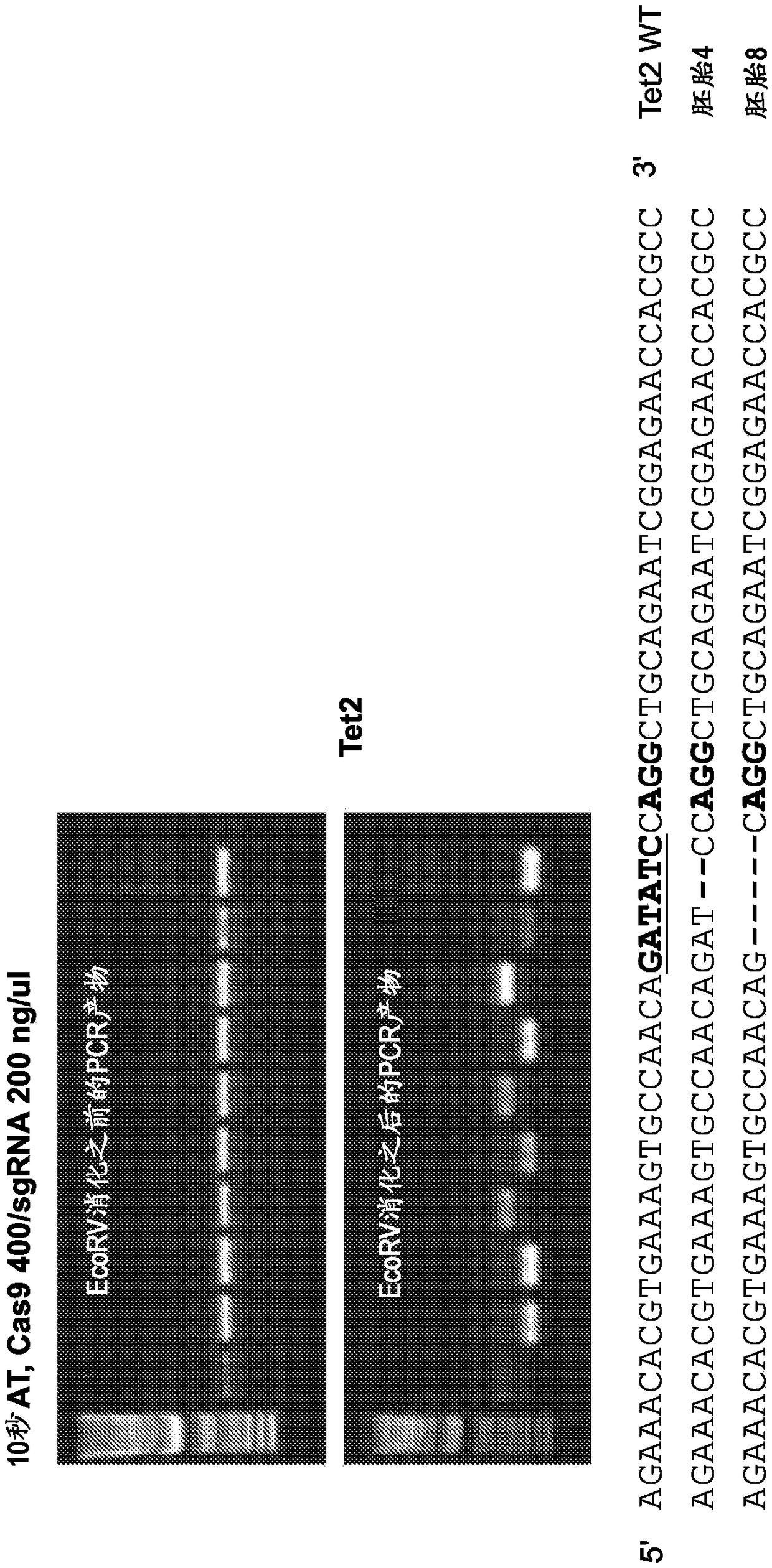
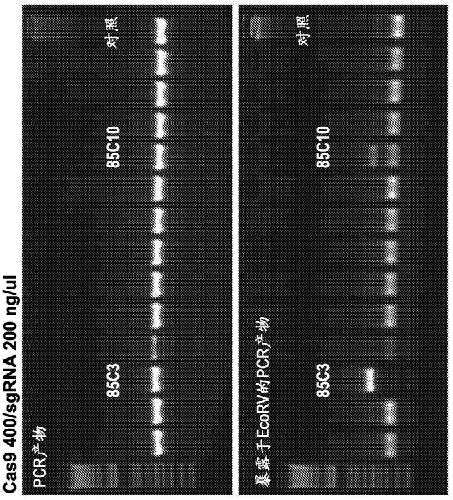
[0264] For this purpose, Tet1 exon 4 and Tet2 exon 3 were selected as targets using the aforementioned guide RNAs (Wang et al., 2013, incorporated herein by reference). The Tet1 and Tet2 systems are convenient in that the Sac1 (Tet1) or EcoRV (Tet2) restriction sites overlap with the PAM (protospacer adjacent motif) proximal sequence. Therefore, restriction fragment length polymorphism (RFLP) analysis can be used to detect mutant alleles using PCR products containing the target site amplified from embryos (Wang et al., 2013).
[0265] In Experiment 6, mouse B6D2F2 zygotes were first treated with AT for 10 s, mixed with 40 / 20 ng / μL or 100 / 50 ng / μL of Cas9 mRN...
Embodiment 3
[0273] The development of the mouse zygote of embodiment 3 electroporation
[0274] The in vitro culture and analysis of zygotes is an important method based on which a large number of parameters related to gene editing technologies can be tested and analyzed with a fast turnaround time and preferably low operating costs. However, when electroporated zygotes were cultured using conventional in vitro culture systems, subsequent embryonic development appeared to be arrested or aborted. Typically, after electroporation of Cas9 mRNA / sgRNA at different concentration ranges (e.g., 50 / 25ng / μL to 1000 / 500ng / μL), at the end of the 3.5-day culture period, embryos progressed to different developmental stages, including 1-cell , 2-cell or 4-cell stage, morula stage and occasionally blastocyst stage (Experiment 8). In contrast, control embryos that were not electroporated reached the blastocyst stage at the end of the same time period, whether or not the control embryos were pretreated wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com