Sucrose phosphorylase mutant and application thereof
A technology of sucrose phosphorylase and amino acid, applied in the field of genetic engineering, to achieve the effect of improving specificity, significant application value, and reducing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, construct recombinant bacteria
[0044] 1. Construction of recombinant bacteria BaSP
[0045] 1. Extract the genomic DNA of Bifidobacterium adolescentis.
[0046] 2. Using the genomic DNA obtained in step 1 as a template, perform PCR amplification with a primer pair composed of F1 and R1, and recover the PCR amplification product.
[0047] F1: 5'-GCCTGGTGCCGCGCGGCAGC CTCGAG atgaaaaacaaggtgcag-3';
[0048] R1: 5'-CAGCTGCAGACCGAGCTCACC CTGCAG tcaggcgacgacaggcggattg-3'.
[0049] 3. Take the PCR amplification product obtained in step 2, perform double digestion with restriction endonucleases XhoI and PstI, and recover the digested product.
[0050] 4. Digest the vector pBAD / HisB with restriction endonucleases XhoI and PstI to recover a vector backbone of about 4000 bp.
[0051] 5. Ligate the digested product of step 3 with the vector backbone of step 4 to obtain the recombinant plasmid pBAD-BaSP.
[0052] According to the sequencing results, the struct...
Embodiment 2
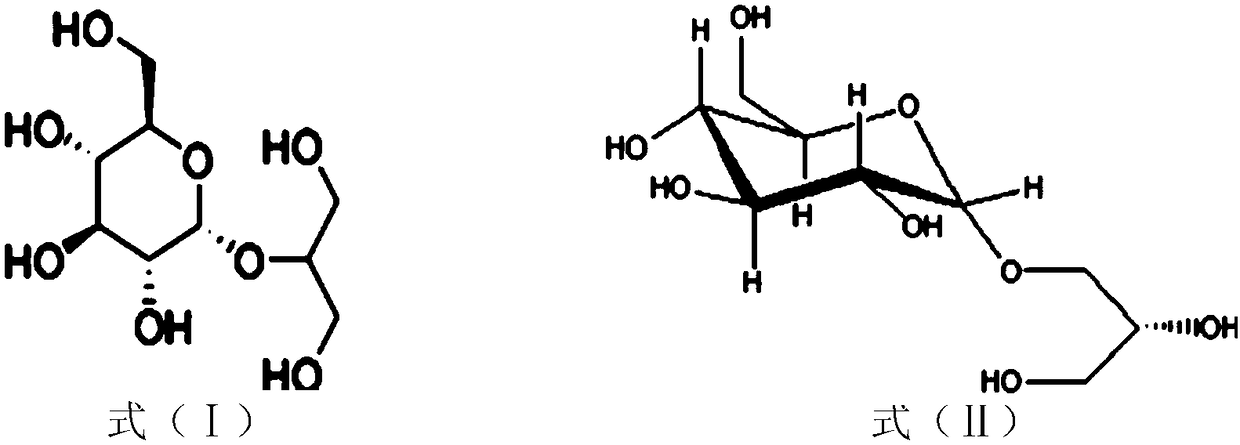
[0068] Embodiment 2, application of recombinant bacteria to prepare 2-glycerol glucoside
[0069] The recombinant bacteria BaSP, recombinant bacteria BaSP / L341D, recombinant bacteria BaSP / L341R or recombinant bacteria pBAD were subjected to the following steps:
[0070] 1. Take a single clone of the recombinant bacteria, inoculate it into liquid LB medium, and culture it with shaking at 37°C and 220rpm until OD 600nm =0.7 (in actual application, OD 600nm =0.6-0.8 can be).
[0071] 2. After completing step 1, add L-arabinose to the culture system so that the concentration in the culture system is 0.2g / 100mL, shake and culture at 30°C and 200rpm for 12 hours.
[0072] 3. After completing step 2, take the entire culture system, centrifuge at 4°C and 6000rpm for 15min, and collect the bacterial precipitate.
[0073] 4. Prepare the reaction system.
[0074] The reaction system is composed of the bacterium precipitate obtained in step 3, glycerol, sucrose and phosphate buffer wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com