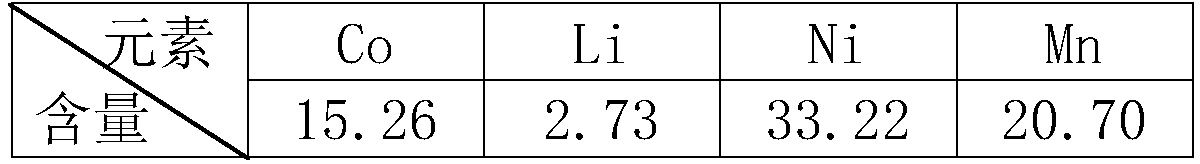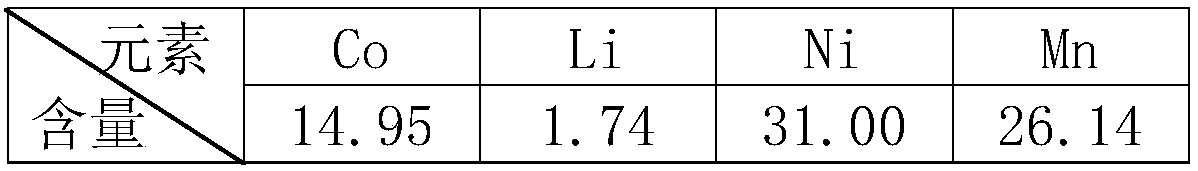Method for leaching valuable metals from waste lithium battery anode material
A technology for waste lithium batteries and cathode materials, which is applied in the field of recycling cathode materials from waste lithium batteries, can solve the problems of high treatment cost, difficult separation, environmental pollution, etc., and achieves the effect of reducing separation and purification operations, low cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Raw material composition (w / %)
[0027]
[0028] Step 1: Dilute the concentrated sulfuric acid to the required concentration, and take 50g of waste material after mixing;
[0029] Step 2: 500ml 2.04mol / L of H 2 SO 4 and 70ml H 2 o 2Add it to step 1, and react for 1.5h at 55±2°C with a stirring rate of 330r / min;
[0030] Step 3: Add 7.0 g of glucose after 1.5 hours of system reaction in Step 2, raise the temperature to 80±2°C, filter and wash with hot water after 1.5 hours of reaction;
[0031] Step 4: Dry and weigh 7.2g of the obtained leaching slag filtered in step 3, and measure the Ni, Co, Mn and Li content in the leaching slag to obtain the leaching rate, as shown in the following table:
[0032] Leaching rate (%)
[0033]
Embodiment 2
[0035] Raw material composition (w / %)
[0036]
[0037] Step 1: Dilute the concentrated sulfuric acid to the required concentration, and take 50g of waste material after mixing;
[0038] Step 2: 500ml 2.51mol / L of H 2 SO 4 and 60ml H 2 o 2 Add it to step 1, and react for 1.5h at 55±2°C with a stirring rate of 330r / min;
[0039] Step 3: Add 10.0 g of glucose after 1.5 hours of system reaction in Step 2, raise the temperature to 80±2°C, filter and wash with hot water after 1.5 hours of reaction;
[0040] Step 4: Dry and weigh 8.6g of the obtained leaching slag filtered in step 3, and determine the content of Ni, Co, Mn and Li in the leaching slag to obtain the leaching rate, as shown in the following table:
[0041] Leaching rate (%)
[0042]
Embodiment 3
[0044] Raw material composition (w / %)
[0045]
[0046] Step 1: Dilute the concentrated sulfuric acid to the required concentration, and take 50g of waste material after mixing;
[0047] Step 2: 500ml 3.24mol / L of H 2 SO 4 and 40ml H 2 o 2 Add it to step 1, and react for 1.5h at 55±2°C with a stirring rate of 330r / min;
[0048] Step 3: Add 20.0 g of glucose after 1.5 hours of system reaction in Step 2, raise the temperature to 80±2°C, filter and wash with hot water after 1.5 hours of reaction;
[0049] Step 4: Dry and weigh 5.8g of the obtained leaching slag filtered in step 3, and measure the Ni, Co, Mn and Li content in the leaching slag to obtain the leaching rate, as shown in the following table:
[0050] Leaching rate (%)
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com