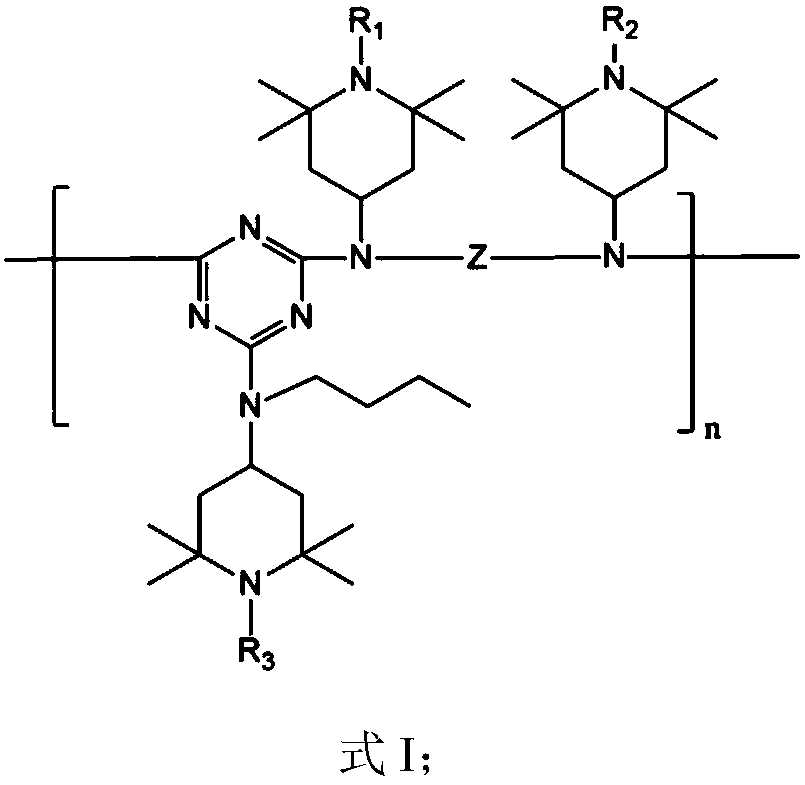
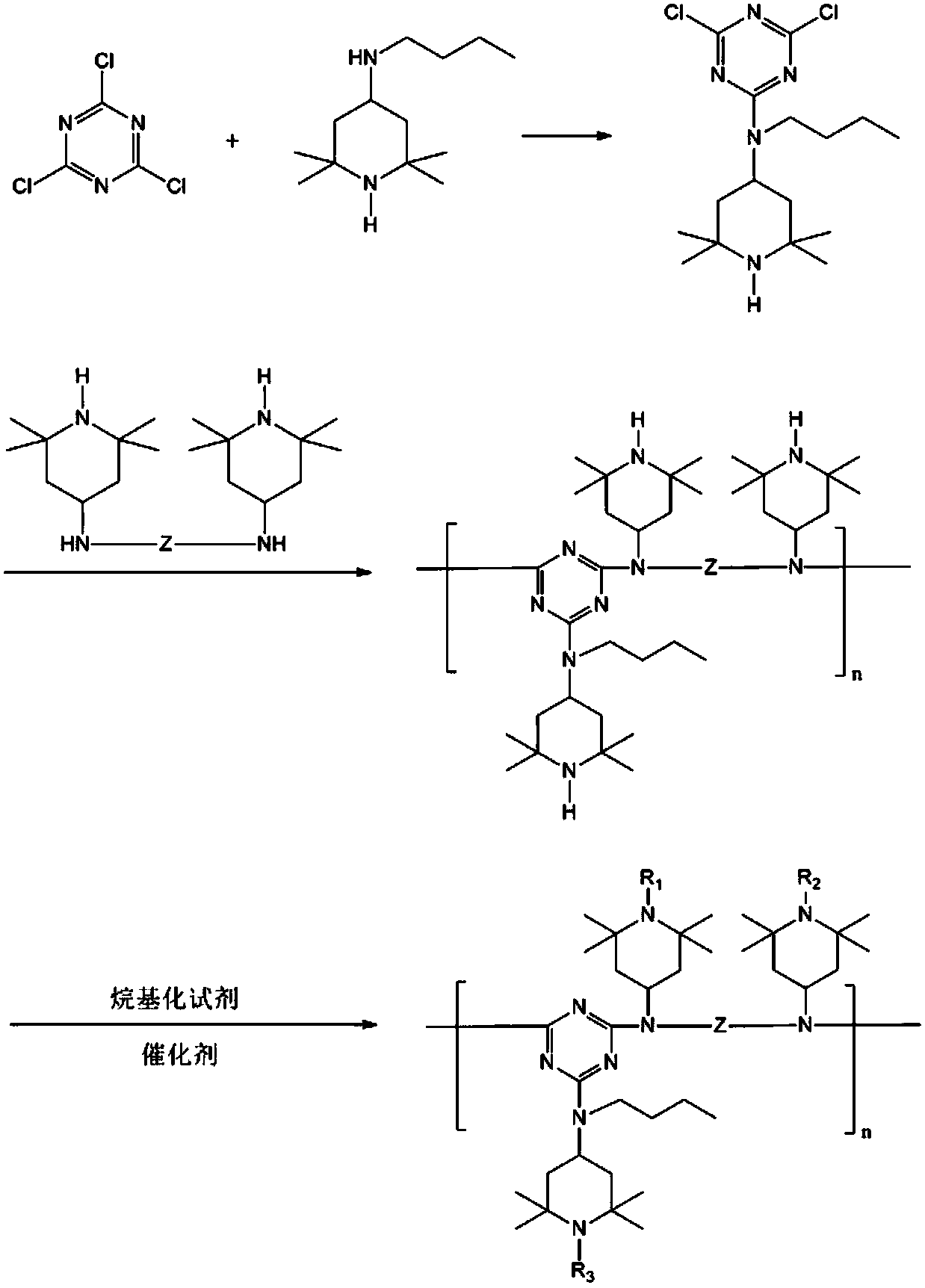
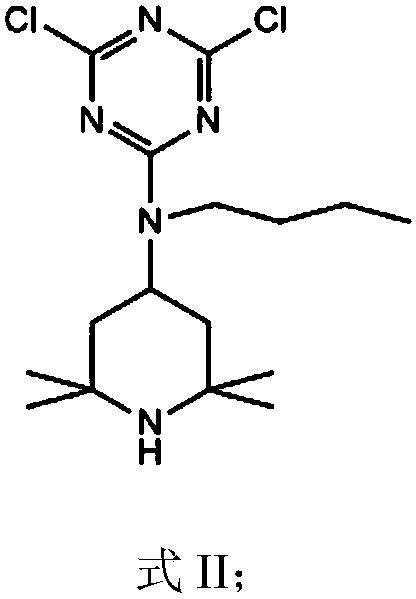
Hindered amine light stabilizer and preparation method thereof
A technology of hindered amine light stabilizer and light stabilizer, which is applied in the field of new hindered amine light stabilizer and its preparation. Narrow distribution, good compatibility, alkalinity reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 hindered amine light stabilizer
[0053] Dissolve 18.4g of cyanuric chloride in 300mL of acetone, and after cooling to 0°C, add 21.2g of N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine (trimer The molar ratio of cyanogen chloride to N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine is 1:1) and 13.8gK 2 CO 3 , Stir for 1h, filter, wash the filtrate with water, and concentrate the organic phase to obtain the intermediate.
[0054] Dissolve the intermediate in 100mL chlorobenzene, add 7.88g N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine (the molar ratio of intermediate to N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine is 1:2), and the reaction After stirring at 30°C for 2h, add 2.76g K 2 CO 3 , then stirred at 50°C for 12h, cooled to room temperature, filtered, the filtrate was washed with water, and the organic phase was concentrated to obtain a polymer.
[0055] Add 150mL bromoethane and 0.9g...
Embodiment 2
[0057] The preparation of embodiment 2 hindered amine light stabilizers
[0058] Dissolve 22.1g of cyanuric chloride in 300mL of dichloromethane, and after cooling to 0°C, add 21.2g of N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine ( The molar ratio of cyanuric chloride to N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine is 1.2:1) and 84g of 10wt% KOH aqueous solution, stirred for 9h, and removed the aqueous phase , The organic phase was concentrated to obtain the intermediate.
[0059] The intermediate was dissolved in 300 mL of toluene, and 9.85 g of N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine ( Intermediate and N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine molar ratio is 1:2.5), reacted in After stirring at 30°C for 2h, add 1.4g of potassium hydroxide, then stir at 80°C for 9h, cool to room temperature, filter, wash the filtrate with water, and concentrate the organic phase to obtain a polymer.
[0060] Add 150mL diethyl ca...
Embodiment 3
[0062] The preparation of embodiment 3 hindered amine light stabilizers
[0063] Dissolve 18.4g of cyanuric chloride in 400mL of toluene, and after cooling to 0°C, add 21.2g of N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine (trimer The molar ratio of cyanogen chloride to N-(2,2,6,6-tetramethyl-4-piperidinyl) n-butylamine is 1:1) and 7.9 g of pyridine, stirred for 1 h, and the organic phase was concentrated to obtain the intermediate.
[0064] The intermediate was dissolved in 100 mL of toluene, and 7.88 g of N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine ( Intermediate and N,N'-bis[N-(2,2,6,6-tetramethyl-4-piperidinyl)]-1,6-hexanediamine molar ratio is 1:2), reacted in After stirring at 30°C for 2h, 1.58g of pyridine was added, then stirred at 50°C for 12h, cooled to room temperature, the reaction solution was washed with water, and the organic phase was concentrated to obtain a polymer.
[0065] Add 150mL bromopropane and 1.9g manganese dioxide to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com