Electrophilically enhanced phenolic compounds for treating inflammatory related diseases and disorders
A compound and disease technology, applied to metabolic diseases, nervous system diseases, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] According to the present disclosure, one or more flavonoids, such as, for example, myricetin, are chlorinated. The compounds may be taken alone, or may be combined in a single dosage form, for example, as capsules, liquid solutions or suspensions, syrups, tablets, powders, and controlled release dosage forms.
[0094] In a preferred embodiment, 1 to 1000 mg of the chlorinated flavonoid is administered orally in capsule form.
Embodiment 2
[0096] Chlorinated flavonoids according to the present disclosure can be administered to various mammalian species in need of such treatment, such as domestic animals, horses, cows, sheep, pigs, dogs, cats, humans, and the like.
[0097] The following example is entitled "Non-GLP Assessment of Antiviral Activity of a Test Compound Against Rhinovirus 14".
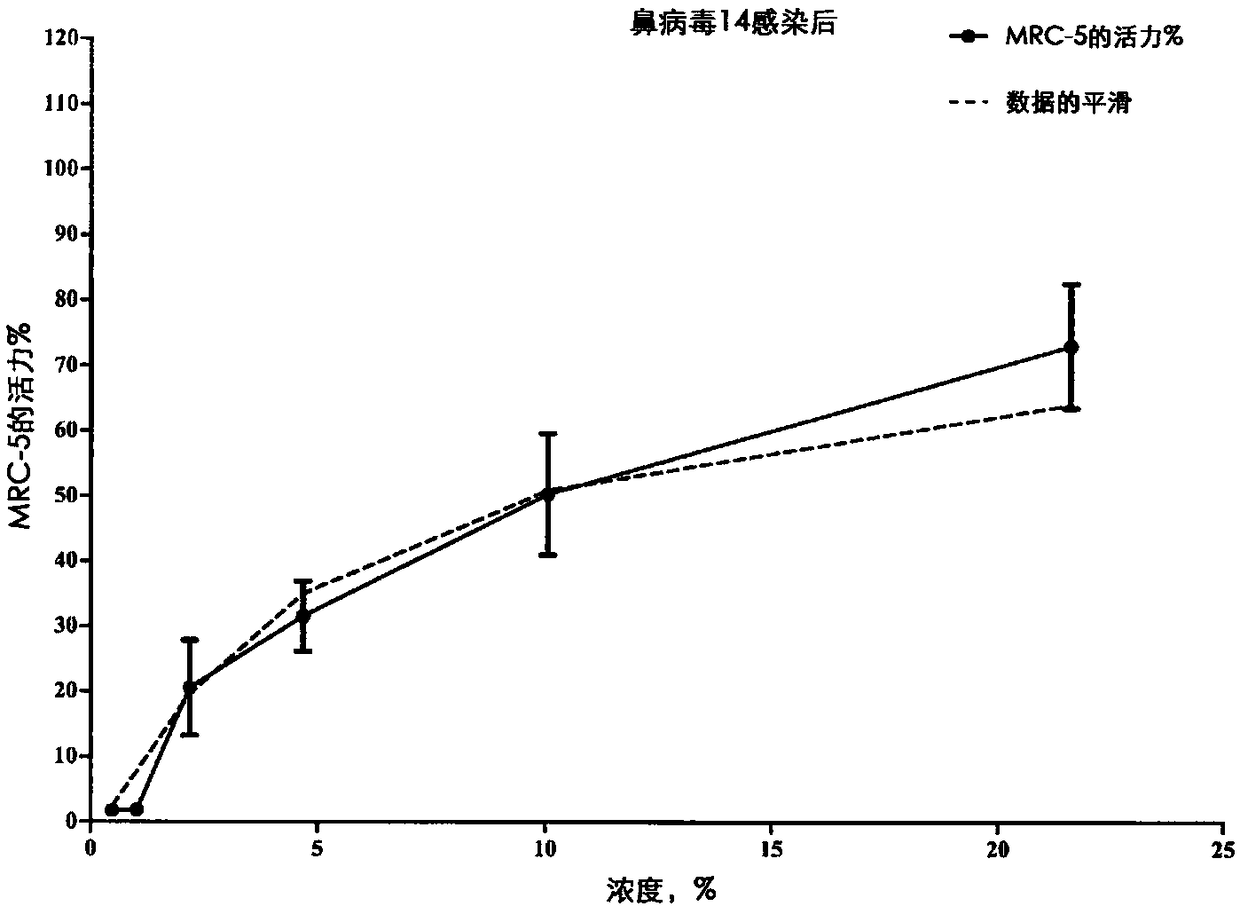
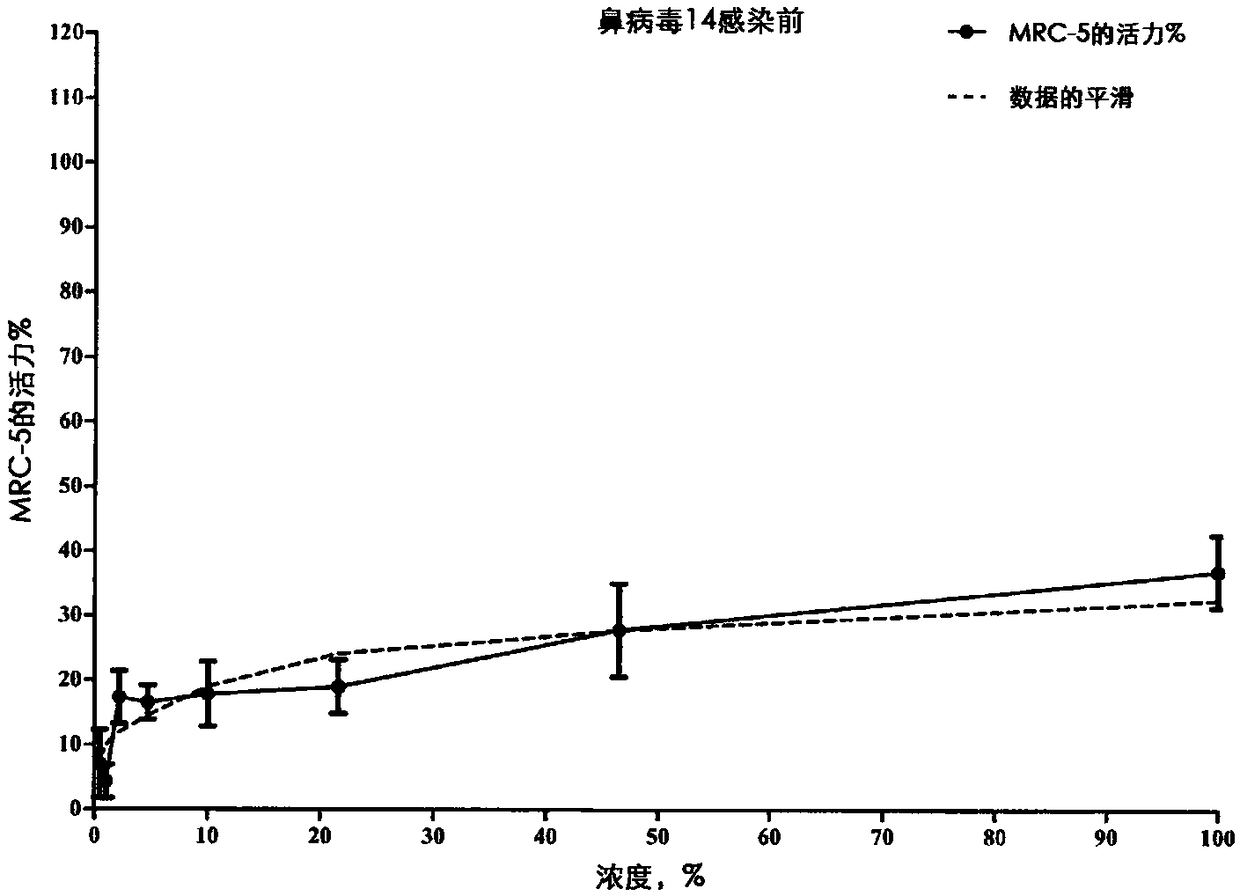
[0098] The test product was prepared as follows: 0.312 g of the test product was dissolved in 10 mL of DMSO to obtain a test product at a concentration of 100 mM; in maintenance medium, a 1 / 1000 dilution of 100 μM test product was prepared to obtain a concentration of 100 uM; Gradation factor of , serial dilutions were performed in maintenance medium. The cytotoxicity of 1 / 1000 dilution of DMSO in culture medium was evaluated. A total of eight different concentrations of the test product were evaluated for their antiviral properties against rhinovirus type 14 strain 1059 (ATCC #VR-284). Post-infection and pre-infection ant...
Embodiment 3
[0142] Analysis of Equivir against influenza virus and dengue virus
[0143] The following examples and experiments have the following objectives:
[0144] In the CPE trial, the antiviral activity of Equivir was determined against three influenza A viruses:
[0145] —A / TX / 36 / 91(H1N1)
[0146] —A / Perth / 265 / 09 (H1N1) and
[0147] —A / HK / 1 / 68(H3N2);
[0148] Determination of cytotoxicity in day 7 MDCK cells;
[0149] Determining the antiviral activity of anti-DENV-2 New Guinea C in dendritic cells (yield reduction assay); and
[0150] Determine the cytokine profile (IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNF -α) (MSD).
[0151] Table 9: Potency Overview
[0152] EC50[μM]
[0153]
[0154] Data affecting viruses in MDCK cells.
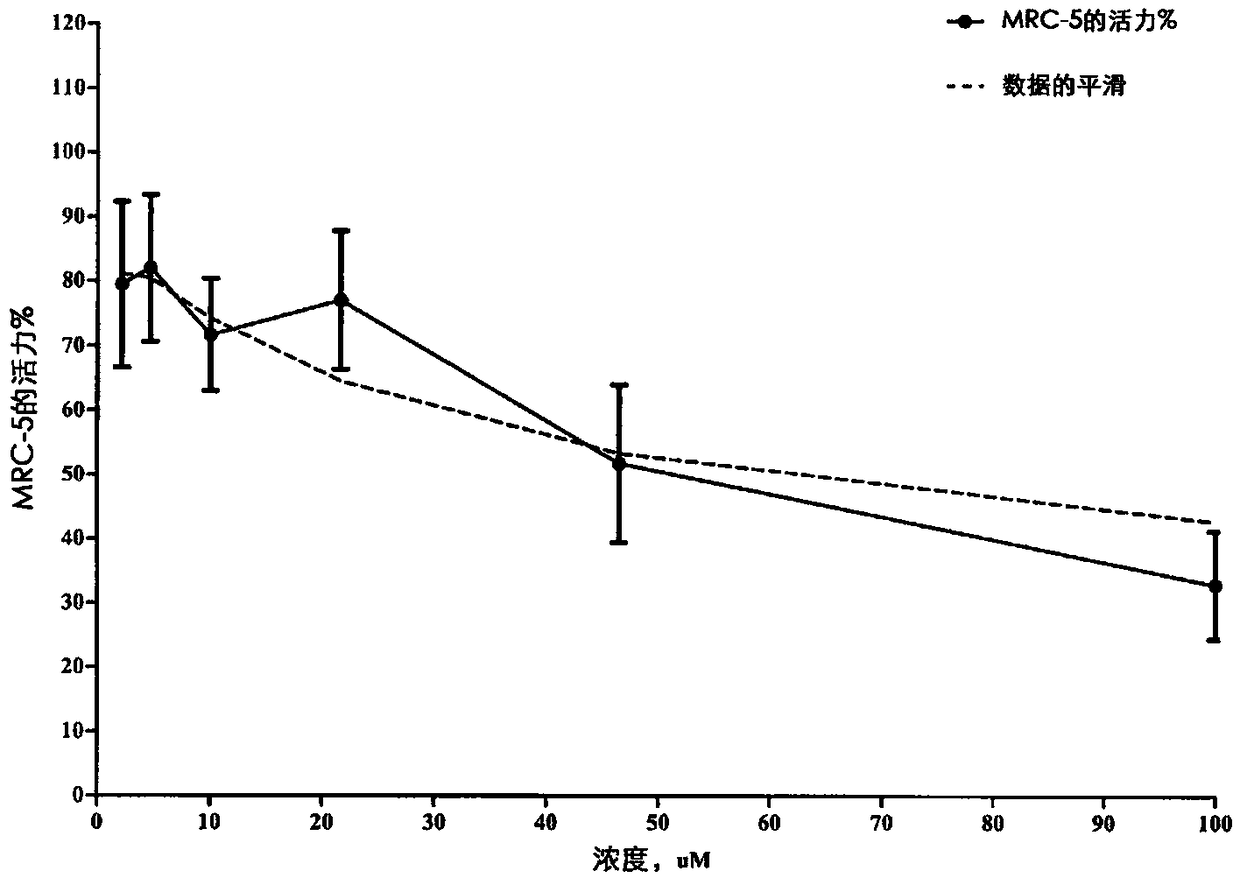
[0155] Data related to influenza virus in MDCK cells are shown in Figure 4-8 middle.
[0156] CPE-based EC50 assay.
[0157] The following experiments were performed as follows:
[0158] Cells were seeded in 96-wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com