Isoliquiritigenin, pharmaceutical composition and application thereof in treatment of diabetic nephropathy
A technology for diabetic nephropathy and isoliquiritigenin, which is applied to isoliquiritigenin, pharmaceutical compositions and their application fields in the treatment of diabetic nephropathy, can solve problems such as immature development and application, and achieves delaying disease progression and deterioration, low cost, Efficacy of alleviating onset and progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0072] Effects of ISL on organ complications in STZ-induced type 1 diabetic mice
[0073] Male C57BL / 6 mice were obtained from the Animal Experiment Center of Wenzhou Medical University. Mice were housed in a thermostated 12-12h diurnal animal room with standard rodent chow and water. Animals underwent more than one week of environmental adaptation before the start of the experiment.
[0074] Male C57BL / 6 mice aged 8-10 weeks were randomly divided into 4 groups: normal control group (ctrl group), diabetes group (STZ-DM1 group), diabetes group combined with ISL (STZ-DM1+ISL group) treatment group (10 , 20mg / kg / d two doses), intragastric administration; every group of 8.
[0075] Low-dose 50mg / kg / d streptozotocin intraperitoneal injection, continuous injection for 5 days to build a type 1 diabetes model, 72 hours later, blood glucose meter to measure fasting blood glucose (fasting for 4-6 hours), blood glucose value ≥ 12mmol / L At that time, a mouse model of type 1 diabetes wa...
experiment example 2
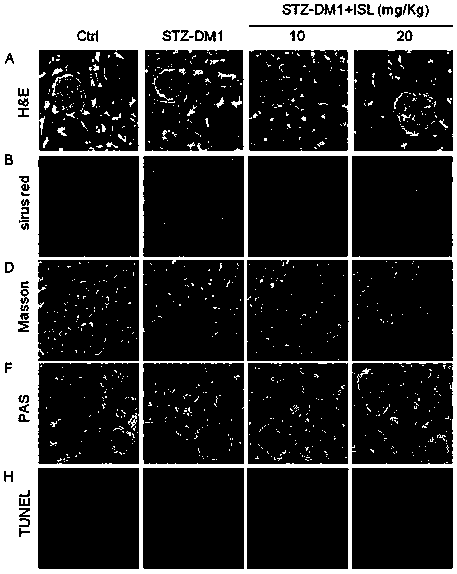
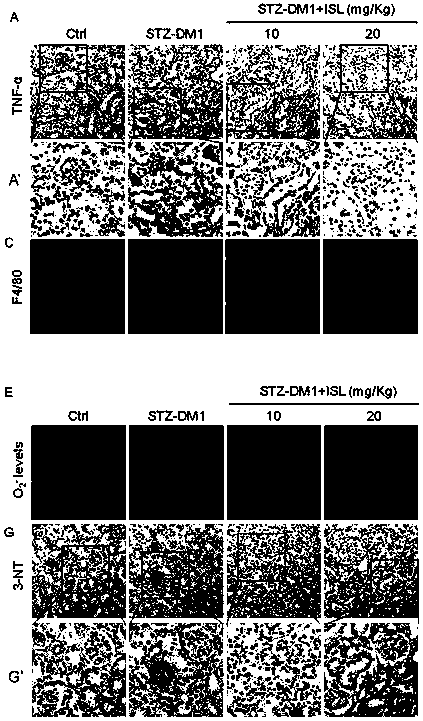
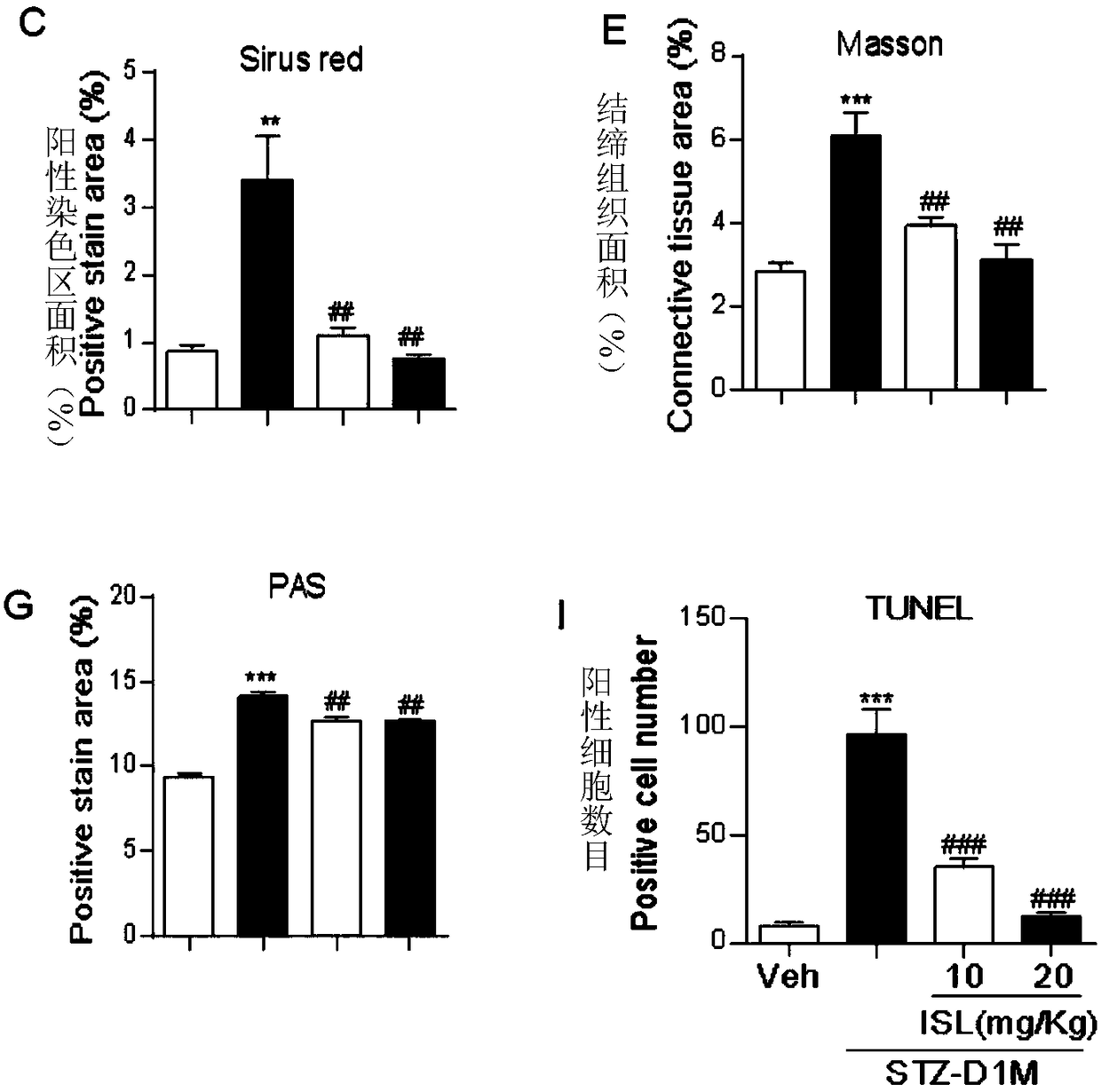
[0085] The kidney tissue obtained in Experimental Example 1 was taken, and the inflammatory factors (TNF-α) and macrophage markers (F4 / 80) in the kidney tissue were detected by immunohistochemistry, and the concentration of reactive oxygen species (ROS) in the kidney was detected by DHE fluorescent dye. Produced and detected oxidative stress-related factor (3-NT) by immunohistochemistry. For experimental data, see image 3 with Figure 4 ,in,
[0086] image 3 Middle A is the effect picture of detecting the inflammatory factor TNF-α in kidney tissue by immunohistochemistry, and A' is the enlarged picture of the boxed part in A, Figure 4 B is image 3 The statistical graph of A. Depend on image 3 A, A' and Figure 4 B shows that ISL can significantly reduce the content of inflammatory factor TNF-α in kidney tissue of STZ-induced type 1 diabetic mice.
[0087] image 3 Middle C is the effect diagram of detecting macrophage markers (F4 / 80) in kidney tissue by immunohis...
experiment example 3
[0093] ISL can alleviate HG-induced renal cell inflammatory response
[0094] The in vitro anti-inflammatory activity of ISL was tested by inhibiting the release of inflammatory factors (TNF-α and IL-1β) and chemokine (MCP-1) in NRK-52E kidney cells stimulated by HG. The specific methods are as follows:
[0095] 1.2×10^6 NRK-52E kidney cells were cultured with DMEM medium at 37°C. After 24 hours, the medium was renewed and pretreated with ISL (final concentration of 10 and 20 μM) for 1 hour, and then treated with high glucose (HG 33mM Glucose) continued to be treated for 6 hours, and the culture fluid was collected to detect the mRNA expression levels of inflammatory factors TNF-α, IL-1β and MCP-1 by RT-qPCR method. Each compound was tested 3 times, and the average value and error value were calculated.
[0096] The in vitro anti-inflammatory and antioxidant activity of ISL was tested by using the method of ISL to inhibit the activation of inflammatory signaling pathway (MAPKs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com