Biocompatible devices, systems, and methods for reducing levels of pro-inflammatory or anti-inflammatory stimulators or mediators in the blood
a technology of pro-inflammatory stimulators and mediators, which is applied in the field of biocompatible devices, systems and methods for reducing the levels of pro-inflammatory or anti-inflammatory stimulators or mediators in the blood, can solve the problems of significant tissue injury, no longer being able to normal immune surveillance, and dysfunctional immune effector cells, so as to prevent incidental inflammatory response conditions, and reduce the population of cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
[0141] In another representative embodiment, the adsorption medium 34 can include particles or beads formed by polymerization of aromatic divinyl compounds, such as p- or m-divinylbenzene or mixtures thereof, or their copolymerization with aromatic monovinyl compounds, such as styrene, methylstyrene, ethylvinylbenzene and vinylbenzylchloride, in the presence of porogens or mixtures of porogens with properties close to those of θ-solvents. The porogens can comprise, e.g., cyclohexane, cyclohexanone and other θ-solvents for polystyrene. Alternatively, the porogens can comprise θ-solvents composed of mixtures of a good solvent for polystyrene, such as toluene, benzene, ethylene dichloride, propylene dichloride, tetrachloroethene, dioxane and methylene dichloride, and a non-solvent for polystyrene, such as aliphatic hydrocarbons, aliphatic alcohols and aliphatic acids.
[0142] Such hypercrosslinked polymeric adsorbents exhibit a combination of micropores, mesopores and macropores. The ad...
example 1
Blood Purification Using an Adsorption Medium to Restore Immunologic Stability
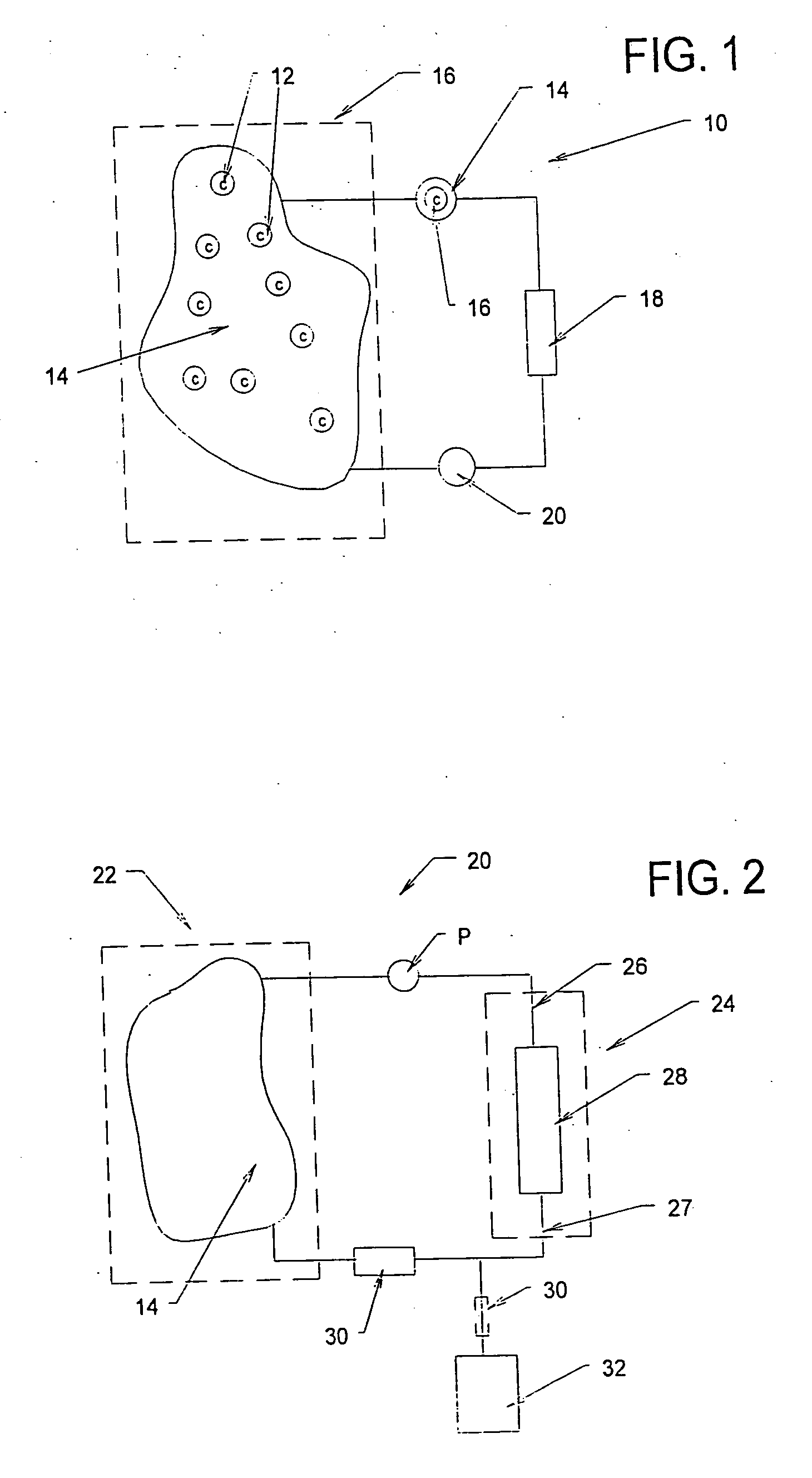
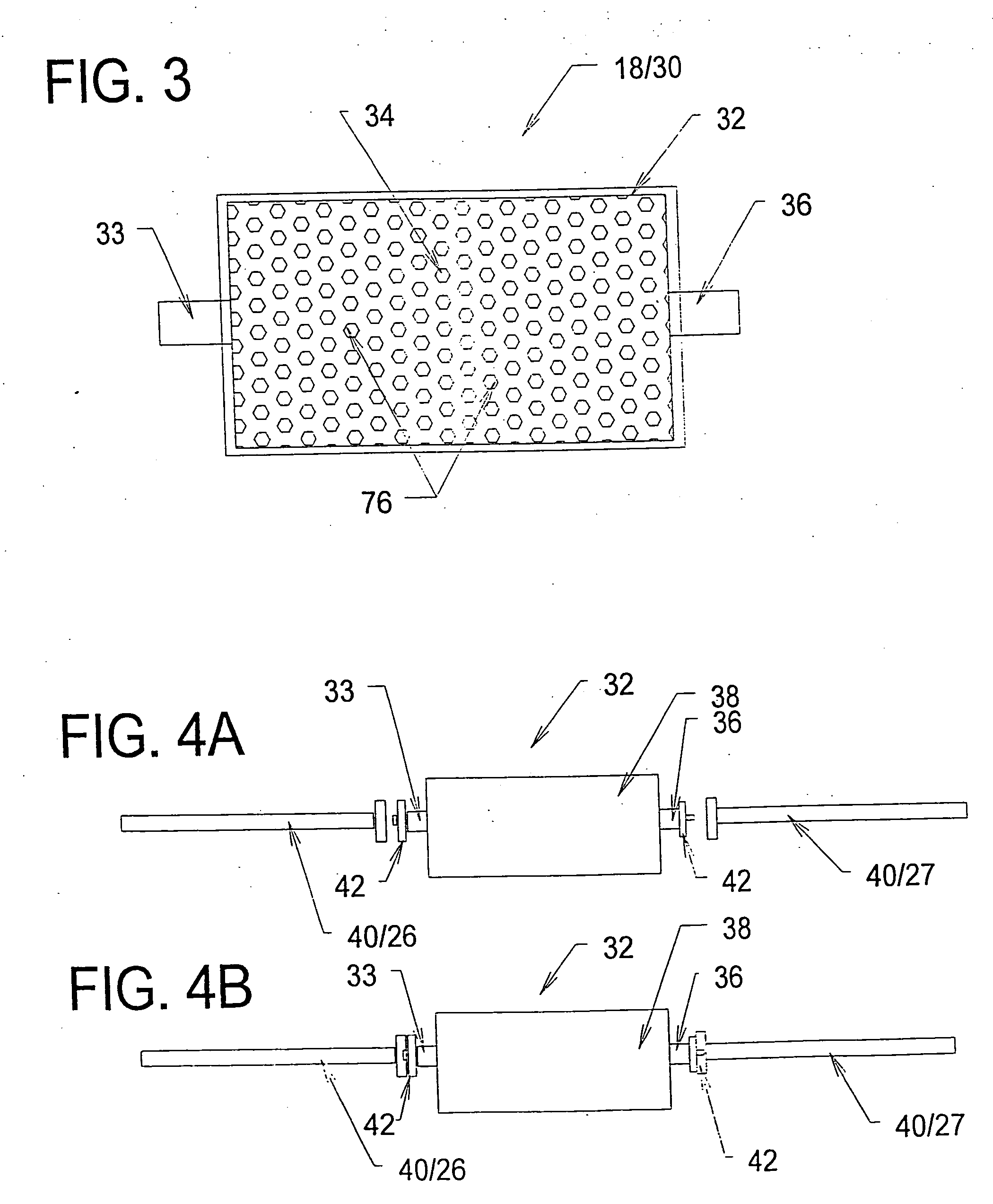
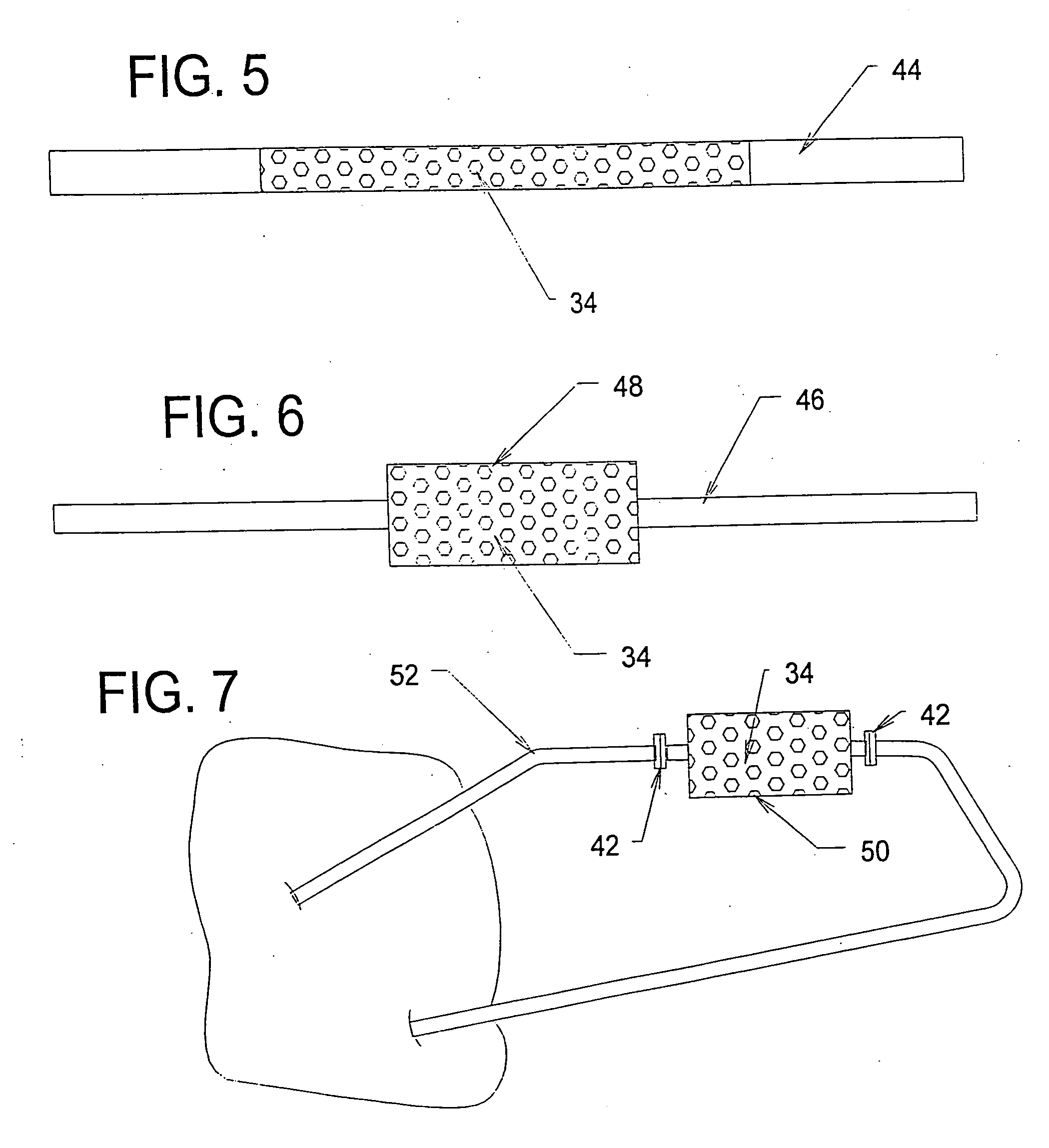
[0198] A study was conducted to demonstrate the ability of a biocompatible adsorption medium to selectively adsorb cytokines (TNF, IL-6, and IL-10) from the blood. The medium comprised particles (as generally shown in FIG. 12) formed of a core of hydrophobic, crosslinked porous divinylbenzene material coated with a thin, permeable biocompatible hydrophilic polyvinylpyrrolidone material. The core material of the particles possessed a mean pore size of about 16 nm. The particles were contained within a housing (as generally shown in FIG. 3) and presented a surface area to blood flow of about 650 sq.mg. The medium was obtained from RenalTech International, New York, N.Y. (BetaSorbm Adsorption Medium).
[0199] The medium was tested in an experiment using in three animals subjected to cecal ligation and puncture (CLP) 18 hrs earlier. The animals tolerated treatment with the medium without difficulty. The cytoki...
example 2
Biocompatibility Index of the Adsorption Medium
[0201] The adsorption medium employed in Example 1 was subjected to the prescribed battery of tests under the biocompatibility index test protocol described above. The blood drawn from six individual healthy donors was subjected to the test protocol and the test results were averaged.
[0202]FIGS. 18 A, 18B, and 18C show the average variations in blood cell counts for red blood cells, white blood cells, and platelets, respectively, incrementally during passage of 25 ml of the blood through the treatment device containing the medium. With respect to red blood cells, white blood cells, and platelets, the maximum difference between the base line (line S.K. / A) and the medium (line S.K. / B) was less than 15%.
[0203]FIG. 19 shows the average variations in PMN elastase concentrations (indicative of leukocyte activation) incrementally during passage of 25 ml of the blood through the treatment device containing the medium. The maximum difference ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrophobicity | aaaaa | aaaaa |
| Biocompatibility | aaaaa | aaaaa |
| Adsorption entropy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com