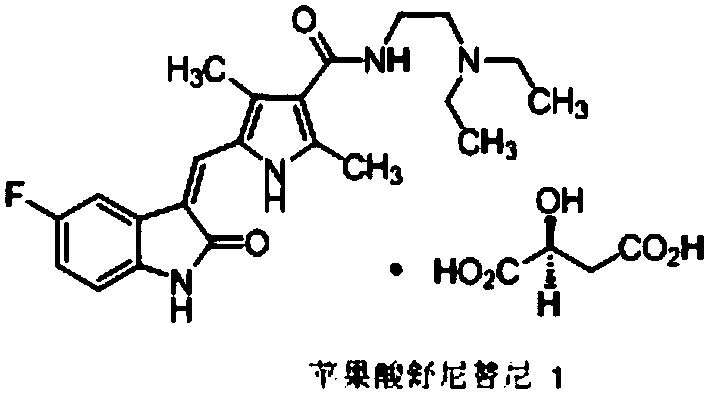
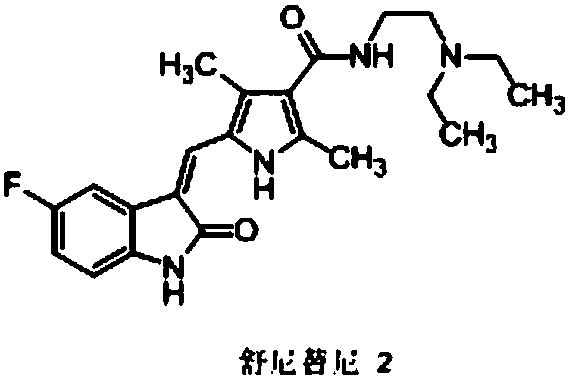
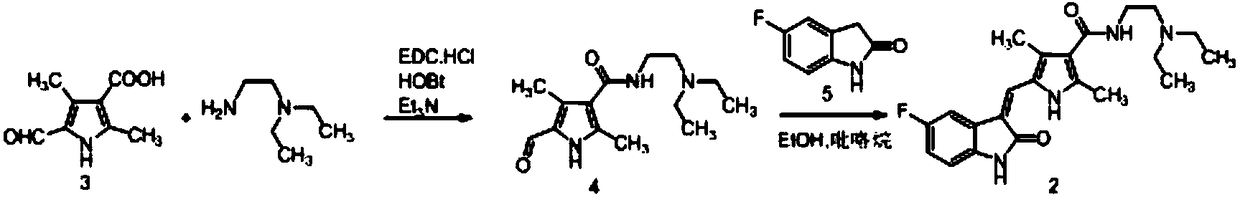
Method for preparing sunitinib
A molar ratio and compound technology, applied in the direction of organic chemistry, can solve the problems of long production cycle, unfavorable large-scale production, slow reaction, etc., and achieve the effects of reduced production power cost, suitable for industrial production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of Sunitinib
Embodiment 2
[0058] Embodiment 2: the selection of optimum experimental condition
[0059] Step 1), 5-fluoroindol-2-one (compound 5, 13g, 1.1eq.) and 2,4-dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid (compound 3, 13g, 1.0eq.) and solvent (39mL) were added to a 100L reaction flask, stirred and heated up according to the experimental requirements, and then catalyst 1 was added to the reaction flask. After the reaction was completed, intermediate compound 12 was obtained.
[0060] Step 2), compound 12 obtained in step 1 was not separated, catalyst 2 / catalyst 3 was added to the reaction solution, N,N-diethylethylenediamine (10.9g, 1.2eq) was added, and the reaction was stirred until the completion of the reaction. A poor solvent (39mL) was added to the solution for crystallization, the stirring was continued until the reaction was complete, and the sunitinib solid was obtained by suction filtration. Orthogonal table L 18 (2×3 7 ) design experiments, the specific results are shown in Table ...
Embodiment 4
[0066] Embodiment 4: Verification of optimal experimental conditions
[0067] Influence of pyrrolidine consumption and experimental temperature in step 1):
[0068] 5-fluoroindol-2-one (compound 5,13g, 1.1eq.) and 2,4-dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid (compound 3,13g, 1.0eq .) and DMF (39mL) were added to a 100L reaction flask, stirred and heated up according to the requirements of the experiment, and then pyrrolidine was added to the reaction flask until the reaction was complete. Add TBTU / DIEA (1.1eq. / 1.7eq.) to the reaction solution, add N,N-diethylethylenediamine (10.9g, 1.2eq.), stir the reaction until complete, add purified water to the reaction solution (39 mL) for crystallization, continued stirring until the reaction was complete, and suction filtered to obtain sunitinib as a solid. The specific results are shown in Table 3.
[0069] The impact of different reaction conditions on the experimental results in table 3
[0070]
[0071]
[0072] It ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com