Construction method and application of TIGIT humanized mouse model
A mouse model, humanized technology, applied in the field of animal genetic engineering and genetic modification to ensure the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Establishment of C57BL / 6-TIGIT humanized mouse model
[0050] 1. Determine the replacement region of the human fragment and the inserted human sequence
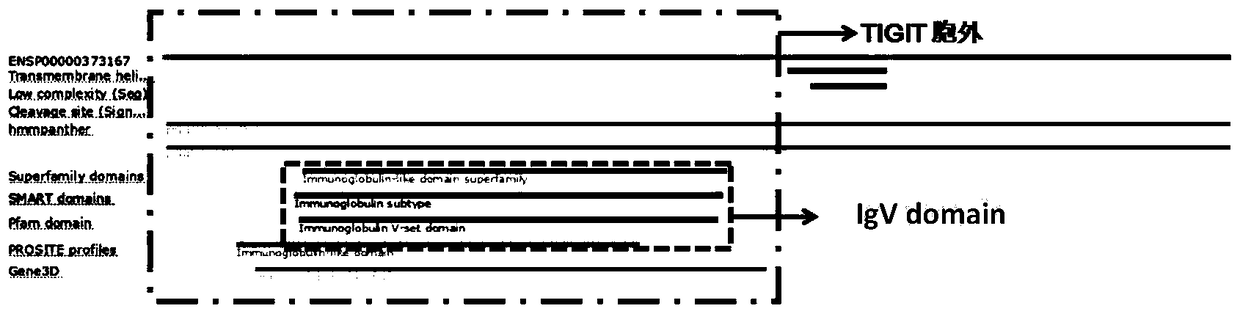
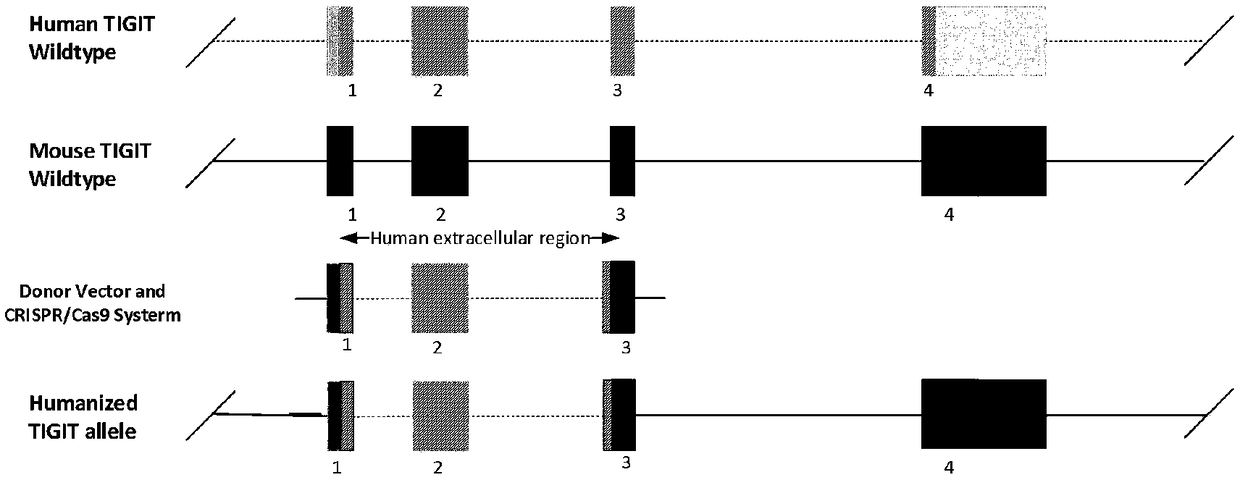
[0051] According to the binding domain of human TIGIT and PVR protein, we selected Exon1, intron1, Exon2, intron2 and Exon3 (transcript NM_173799.3) of human TIGIT gene sequence (Gene ID: 201633) to replace Exon1 and intron1 of mouse TIGIT , Exon2, intron2 and Exon3 (transcript NM_001146325.1), the transmembrane region and intracellular region of mouse TIGIT are retained, and the replacement strategy is shown in the figure figure 2 shown. The chimeric sequence of the replaced protein is as follows, the underlined part is the human replacement region:
[0052] MRWCLLLIWAQGLRQAPLASG MMTGTIETTGNISAEKGGSIILQCHLSSTTAQVTQVNWEQQDQLLAIC NADLGWHISPSFKDRVAPGPGLGLTLQSLTVNDTGEYFCIYHTYPDGTYTGRIFLEVLESSVAEHGARFQIP LGGTMAAAVLGLICLMVTGVTVLARKKSIRMHSIESGLGRTEAEPQEWNLRSLSSPGSPVQTQTAPAGPCGEQAEDDYADPQEYFNVLSYRSLESFIAVSKT...
Embodiment 2
[0087] Example 2 Human TIGIT expression and immune system verification in B6-hTIGIT mice
[0088] The expression of TIGIT homozygous mice was analyzed by flow cytometry and the immune cell population was checked. After analysis, the mice that could successfully express the humanized gene and did not cause obvious abnormalities in the immune system were available for tumor drug efficacy experiments.
[0089] 1. Protein expression detection
[0090] Thymus or spleen was selected as the main expression tissue of TIGIT, and the expression of TIGIT protein in the tissue was detected.
[0091] TIGIT protein flow detection method:
[0092] a) Sampling: Spleens were cut from B6-hTIGIT homozygous mice and C57BL / 6 background mice (see mouse information list), weighed, and placed in C-tubes.
[0093] b) Digestion: Peripheral blood was protected from light at room temperature and washed once with FACS buffer; spleen and thymus were digested with enzyme solution (PBS containing Ca, Mg+2%...
Embodiment 3B6
[0112] Example 3B6-hPD1 / hTIGIT mouse expression and functional verification of human TIGIT and PD1
[0113] 1. Mating PD1 pure and humanized mice with TIGIT humanized mice to obtain PD1 heterozygous TIGIT heterozygous humanized mice, male mice and PD1 pure and humanized mice were subjected to IVF to obtain a large number of PD1 pure and TIGIT heterozygous humanized mice, when the PD1 pure and TIGIT heterozygous humanized mice reach the appropriate age, the siblings are mated to obtain PD1 and TIGIT double pure and humanized mice, identification scheme and identification results As follows, the obtained PD1 and TIGIT double pure and humanized mice are numbered 148, 155, 161, 168, 172, 177-180, 182, 194#.
[0114] PCR Primer Information
[0115]
[0116]
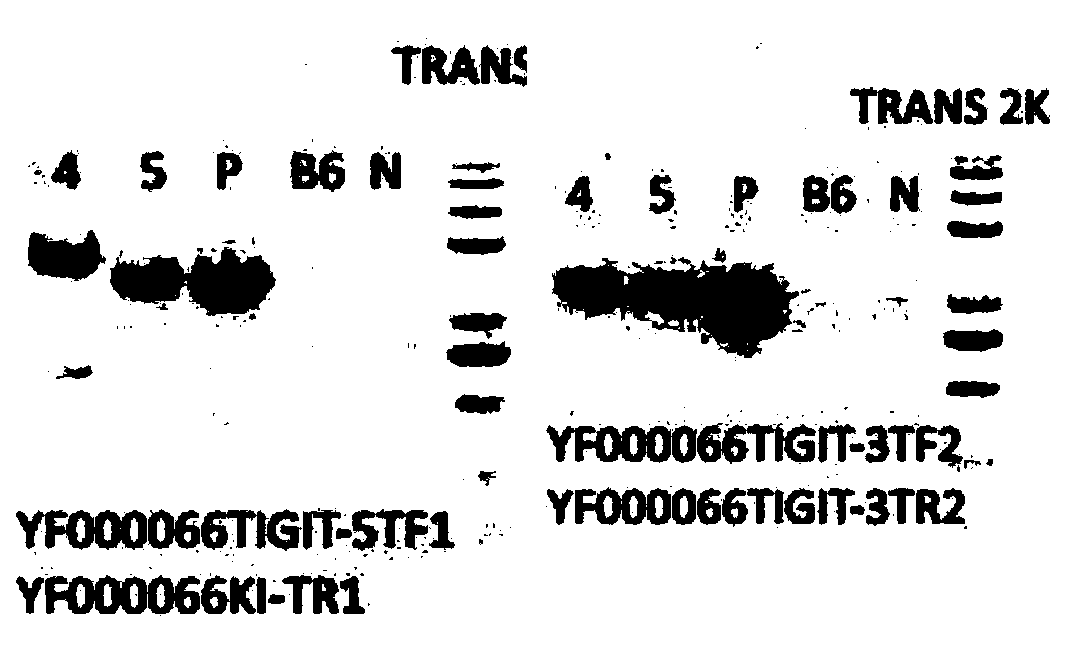
[0117] Figure 8 – Figure 11 The electrophoresis identification diagram during the preparation of B6-hPD1 / hTIGIT mice is shown, in which Figure 9 The identification results of TIGIT-KI-target 5-end and 3-end are sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com