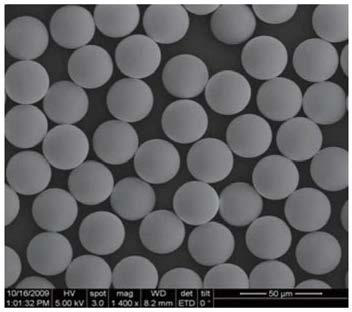
Method for separating and purifying sugammadex
A technology for separating and purifying sugammadex, which is applied in the field of high-efficiency separation and purification of sugammadex, can solve problems such as harm, and achieve the effects of cost reduction, simple and convenient operation, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
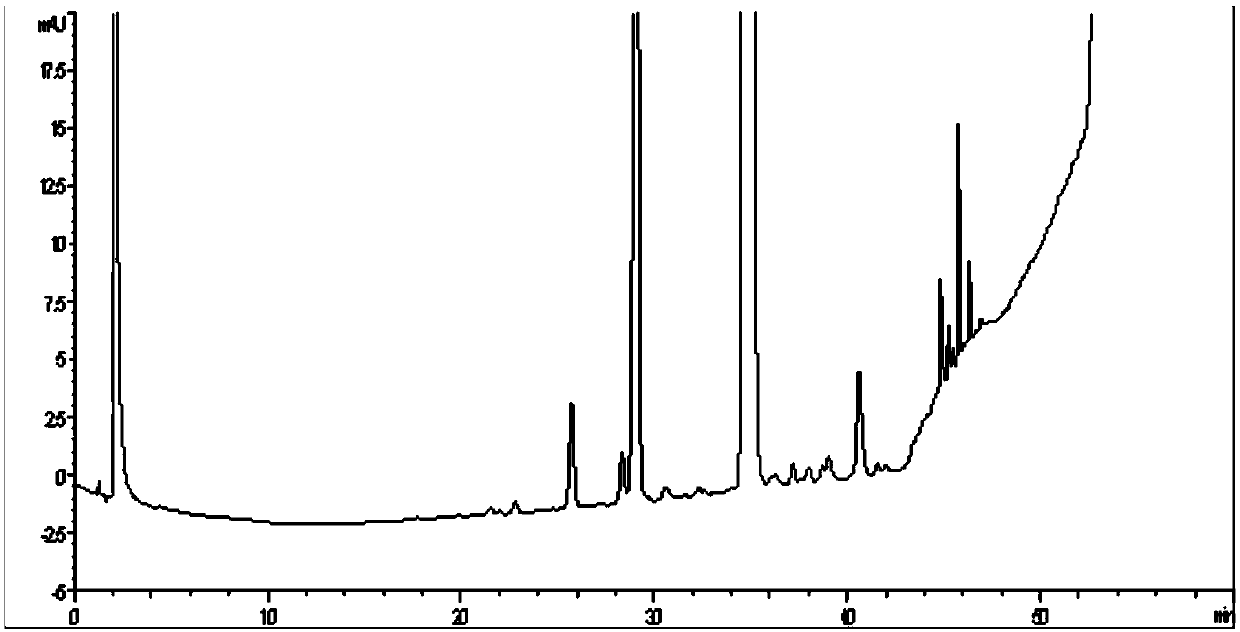
[0038] The crude extract of sugammadex with a purity of 93.11% was taken and dissolved in water, and the content of sugammadex in the solution was 50 mg / mL. After the solution was clarified, it was filtered through a filter membrane with a pore size of 0.45 μm, and the filtrate was collected for later use. A chromatographic column of 4.6 × 250 mm was used, and UniDEAE-30S microspheres (produced by Suzhou Nawei Technology Co., Ltd.) were used as the packing material of the chromatographic column, and the packing volume was 4.2 ml. Gradient elution was performed with 20mM sodium acetate solution (phase A) and 20mM sodium acetate + 1M NaCl (phase B) as the mobile phase. Perform pre-column pretreatment on the chromatographic column, first remove impurities with 50% phase B, and then equilibrate with 100% phase A. The flow rate was controlled at 0.4ml / min. The gradient elution process is as follows: wash with 100% A phase for 15 minutes first, then increase the elution concentrat...
Embodiment 2
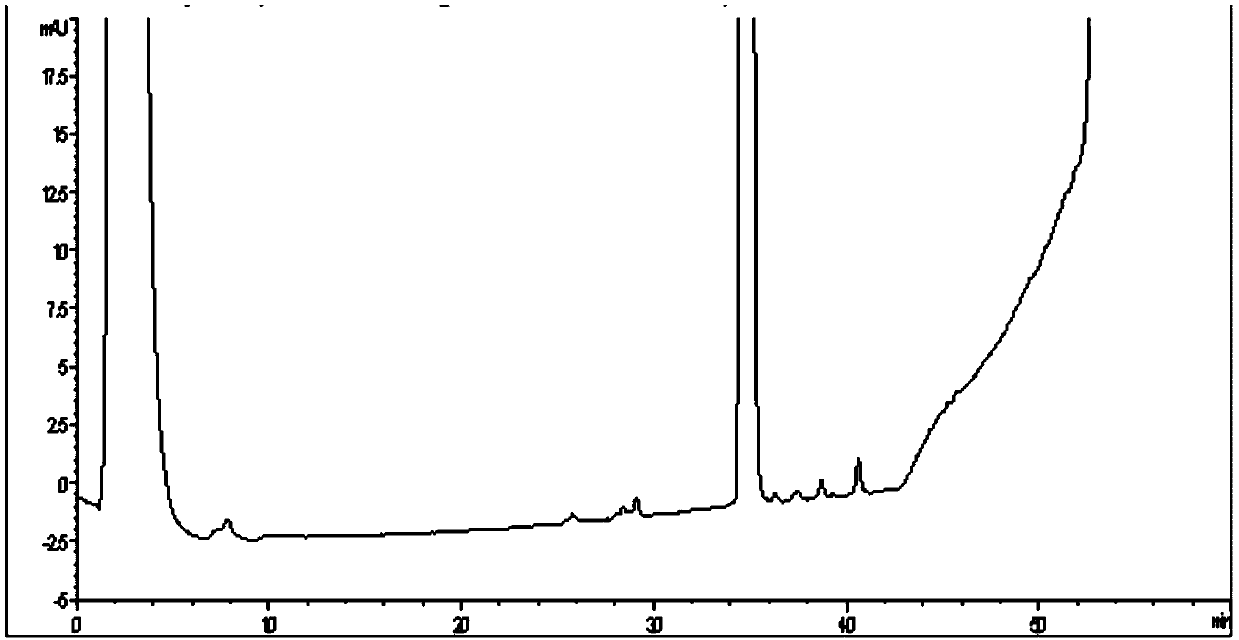
[0041] The crude sugammadex extract with a purity of 92.55% was taken, dissolved in water, and the sugammadex content in the solution was 50 mg / mL. After the solution was clarified, it was filtered through a filter membrane with a pore size of 0.45 μm, and the filtrate was collected for later use. A chromatographic column of 4.6×250mm was used, and UniQ-30S microspheres were used as the column filler, and the column volume was 4.2ml. Use 20mM sodium acetate solution (phase A) and 20mM sodium acetate + 1MNaCl (phase B) as the mobile phase for gradient elution, and perform pre-column pretreatment on the chromatography column, first use 50% phase B to remove impurities, and then use 100% %A phase for equilibrium. The flow rate was controlled at 0.4ml / min. The gradient elution process is as follows: wash with 100% A phase for 15 minutes, then increase the concentration gradient from 0% B to 4% B within 15-20 minutes, and increase the concentration gradient from 4% B to 8% within...
Embodiment 3
[0043]The crude sugammadex extract with a purity of 92.55% was taken, dissolved in water, and the sugammadex content in the solution was 50 mg / mL. After the solution was clarified, it was filtered through a filter membrane with a pore size of 0.45 μm, and the filtrate was collected for later use. A chromatographic column of 4.6×250 mm was used, and UniDEAE-30S microspheres were used as the column filler, and the column volume was 4.2 ml. Use 20mM sodium acetate solution (phase A) and 20mM sodium acetate + 1MNaCl (phase B) as the mobile phase for gradient elution, and perform pre-column pretreatment on the chromatography column, first use 50% phase B to remove impurities, and then use 100% %A phase to equilibrate. The flow rate was controlled at 0.4ml / min. The gradient elution process was that 10% B was increased to 40% B for 20 column volumes. Collect the solution of the target peak in sections, and summarize the components that meet the requirements. After high performance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com