Rabdosia serra formula granule used for treating acute jaundice hepatitis and preparation method thereof
A technology for formula granules and acute jaundice, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, plant raw materials, etc., to achieve the effects of easy storage, uniform particle size, and convenient implementation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method of Xihuangcao formula granule of the present invention comprises the following steps:
[0022] (1) pulverizing the dried Xihuang herbal medicinal material to obtain a powder with a particle size of 40-100 mesh;
[0023] (2) Soak the obtained powder in 2-5 times the volume of hot water;
[0024] (3) filter, the filter cake obtained is mixed with the aqueous ethanol solution of 15-20 times of feeding amount;
[0025] (4) ultrasonic extraction 2-3 times;
[0026] (5) filter, and the filtrate is concentrated under reduced pressure to a concentrated solution whose relative density is 1.05-1.15;
[0027] (6) mixing the concentrate with pharmaceutically acceptable adjuvants;
[0028] (7) Freeze-drying, collecting the dry cream powder, pulverizing through an 80-mesh sieve, and mixing in a mixing tank;
[0029] (8) The obtained Xihuangcao ointment was granulated, and the granules were sequentially sized through a 20-mesh sieve and a 50-mesh sieve to ob...
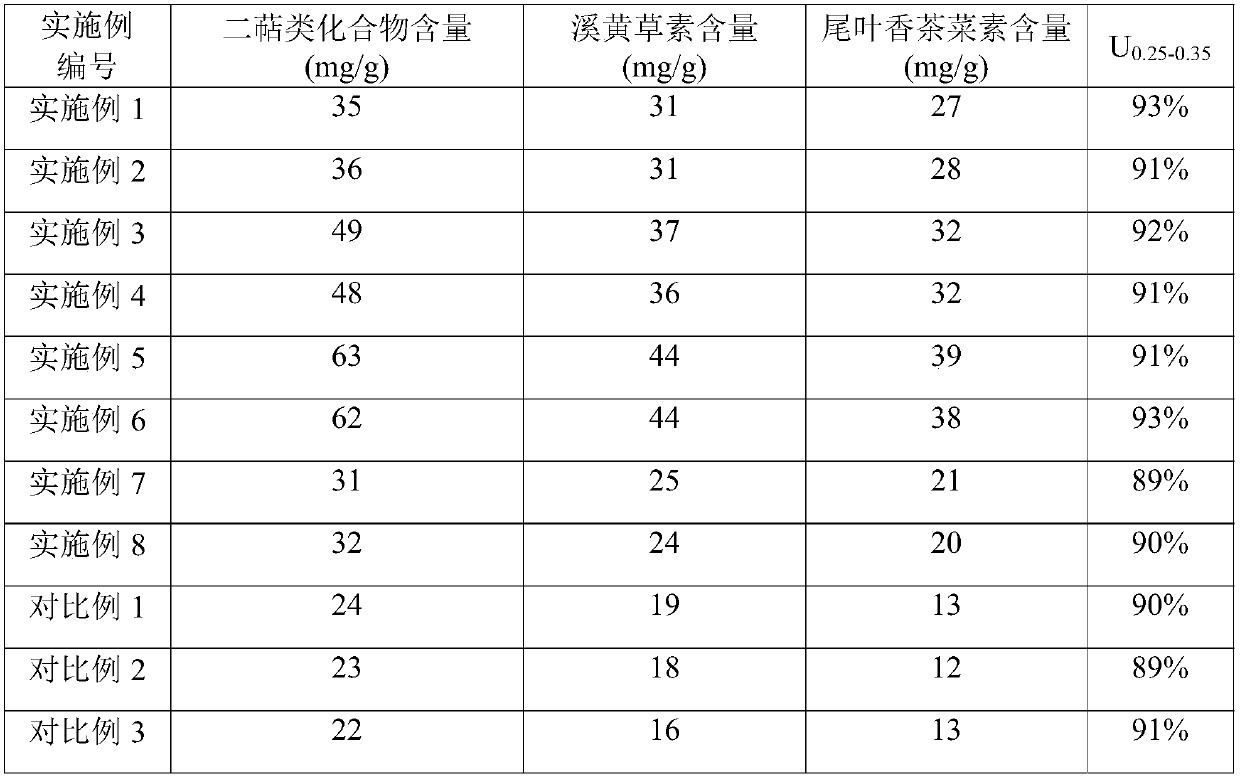
Embodiment 1
[0045] (1) Take 1 kg of dried Xihuang herb, crush it to 80 mesh, soak it in hot water (50°C) with 3 times the feeding amount for 2 hours, filter it with 200 mesh stainless steel wire mesh, and mix the obtained filter cake with 18 times the feeding amount Aqueous ethanol solution (concentration is 45%) is mixed, ultrasonically extracts 3 times under the ultrasonic frequency of 90kHz, extracts 45 minutes each time, filters with 200 order stainless steel screens, and the filtrate is concentrated under reduced pressure to the concentrated solution that relative density is 1.10;
[0046] (2) Add 100g of a mixture of starch, lactose and dextrin (the weight ratio of the three is 1:0.25:1) to the concentrated solution for mixing, freeze-dry, collect the dry cream powder, crush it through an 80-mesh sieve, and mix it in a mixing tank ;
[0047] (3) Take Xihuangcao paste powder, sieve and granulate, and granulate the granules with a 20-mesh sieve and a 50-mesh sieve in turn to obtain Xi...
Embodiment 2
[0049] (1) Take 1 kg of dried Xihuang herb, crush it to 100 mesh, add 4 times the amount of hot water (45°C) to soak for 3 hours, filter with 200 mesh stainless steel wire mesh, and mix the obtained filter cake with 16 times the amount of feed Aqueous ethanol solution (concentration is 40%) is mixed, ultrasonically extracts 3 times under the ultrasonic frequency of 90kHz, extracts 45 minutes each time, filters with 200 order stainless steel screens, and the filtrate is concentrated under reduced pressure to the concentrated solution that relative density is 1.12;
[0050] (2) Add 100g of a mixture of starch, lactose and dextrin (the weight ratio of the three is 1:0.25:1) to the concentrated solution for mixing, freeze-dry, collect the dry cream powder, crush it through an 80-mesh sieve, and mix it in a mixing tank ;
[0051] (3) Take Xihuangcao paste powder, sieve and granulate, and granulate the granules with a 20-mesh sieve and a 50-mesh sieve in turn to obtain Xihuangcao fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com