Composition, screening model and screening method for screening anti-tuberculosis drugs
A technology of culture and aspartic acid, applied in the field of medicine, can solve problems such as difficult to break through MTBASD, and achieve the effect of high-throughput screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Cloning and expression of embodiment 1 recombinant aspartate semialdehyde dehydrogenase ASD
[0064] (1) Cloning of asd gene and construction of expression vector
[0065] Primers were designed according to the ASD gene sequence published by NCBI (GenBank No. 9 885118): asdh F (5'-TTTT GAATTC ATGGGCCTGTCAATAGGGATC-3') and asdh R (5'AAAA AAGCTT TCACAAGTCGGCGGTCAGC) was used to amplify the ASD gene using the MTB genome as a template. Underlined parts are EcoR I and Hind III restriction enzyme sites. The reaction system is:
[0066]
[0067] PCR SuperMix was purchased from Beijing Quanshijin Biotechnology Co., Ltd., and primers were synthesized by Beijing Huada. The reaction procedure is:
[0068]
[0069] The PCR product was purified using a gel recovery kit (Beijing Quanshijin Biotechnology Co., Ltd.), the purified product and plasmid pET28a(+) were digested with EcoR I and Hind III (Dalian TaKaRa Company) respectively, and the digested fragments were heat-s...
Embodiment 2
[0074] Embodiment 2 Purification of recombinant aspartate semialdehyde dehydrogenase
[0075] Cell disruption: Suspend the collected cells with lysis buffer (20mM Tris-HCl, 500mM NaCl, 10mM imidazole, 1mM DTT, pH 8.0) into a 50mg / mL bacterial solution; use a pressure breaker pre-cooled to -15°C The cells were broken three times under the pressure of 800 bar; the broken cell solution was centrifuged at 10000 g for 20 min to remove insoluble matter, and then filtered with a 0.45 μm filter.
[0076] Protein purification: using The explorer system was used for protein purification, using 1mL prepacked column HiTrapChelating HP as the purification medium.
[0077] Elute with a buffer containing 20mM Tris-HCl, 500mM NaCl, 400mM imidazole and 1mM DTT, pH 8.0 according to the following procedure:
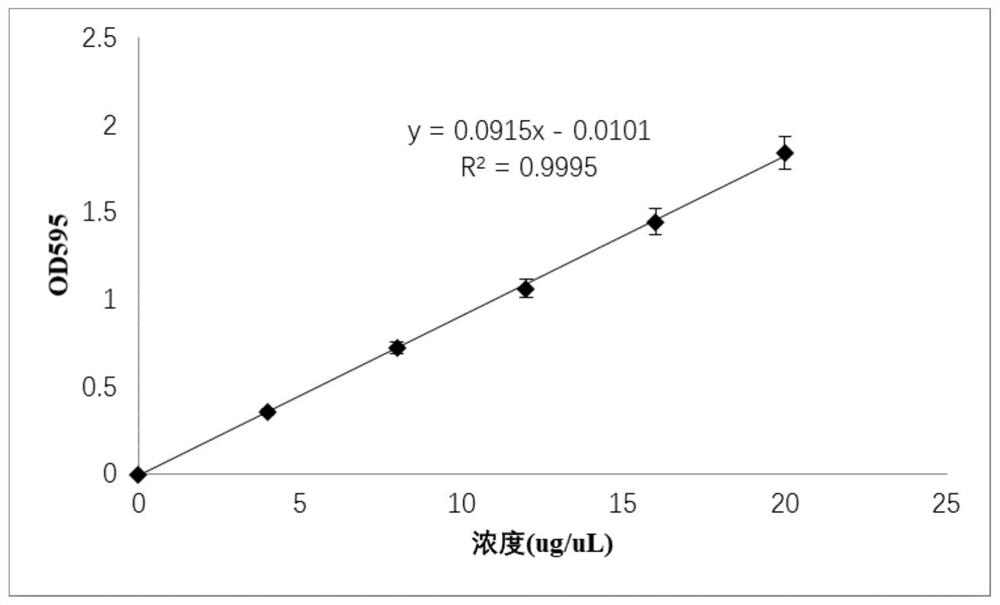
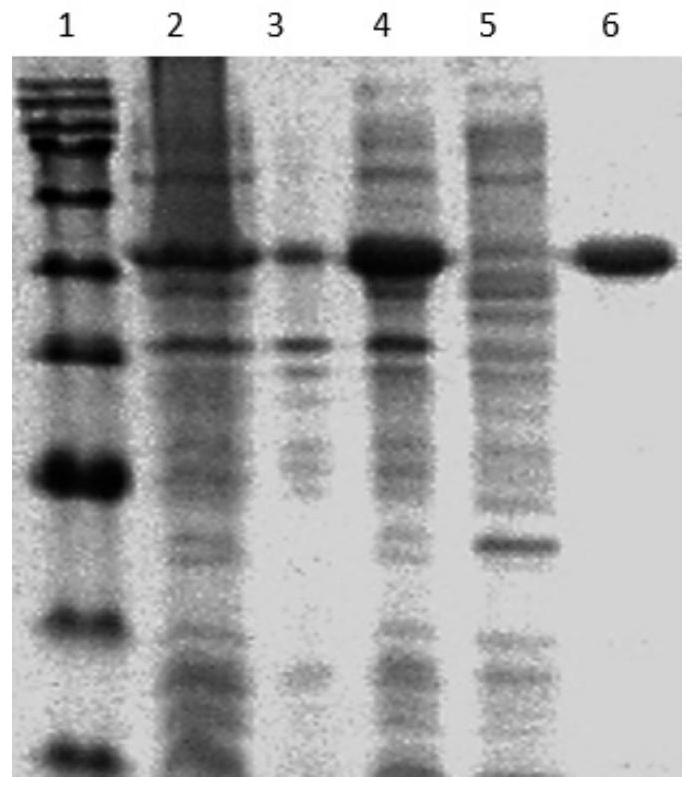
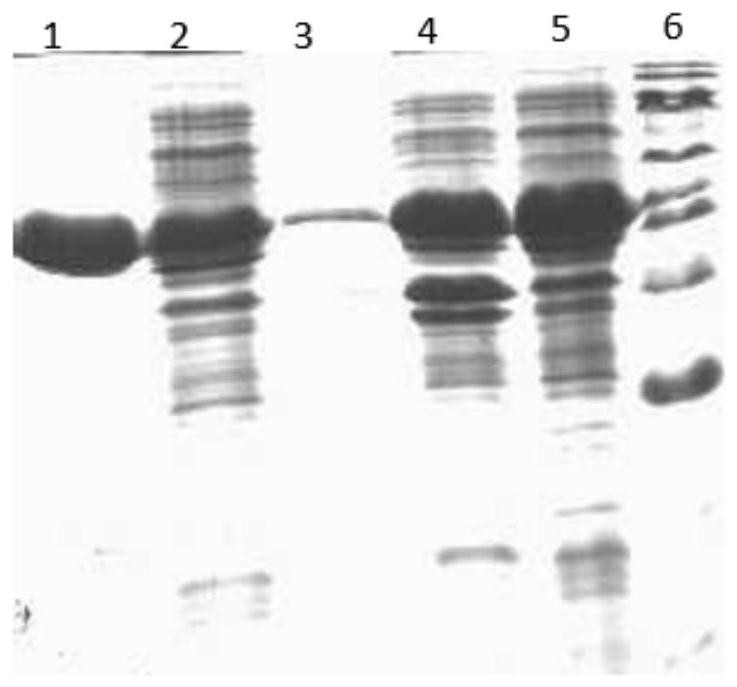
[0078] The eluent concentration was 0-100%, the elution time was 10 min, the collection volume was set at 1 mL, and the purification effect was detected by SDS-PAGE.
[0079] Protein de...
Embodiment 3
[0093] Example 3 Expression and Purification of Recombinant Escherichia coli Type III ASK (Lys C)
[0094] (1) Cloning of LysC gene and construction of expression vector
[0095] Primers were designed according to the LysC gene sequence published by NCBI (GenBank No. 948531): lysC F(5'-TTTT GAATTC ATGTCTGAAATTGTTGTCT-3') and lysC R(5'-AAAA AAGCTT TTACTCAAACAAATTACTA-3') was used to amplify the LysC gene using the Escherichia coli genome as a template. Underlined parts are EcoR I and Hind III restriction enzyme sites. The reaction system is:
[0096]
[0097] PCR SuperMix was purchased from Beijing Quanshijin Biotechnology Co., Ltd., and primers were synthesized by Beijing Huada. The reaction procedure is:
[0098]
[0099] The PCR product was purified using a gel recovery kit (Beijing Quanshijin Biotechnology Co., Ltd.), the purified product and plasmid pET28a(+) were digested with EcoR I and Hind III (Dalian TaKaRa Company) respectively, and the digested fragment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com