Deep-sea-derived strains and their encoded β-galactosidase genes and applications
A technology of galactosidase and strains, applied in the fields of application, glycosylase, genetic engineering, etc., can solve the problem of low activity and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 produces the screening of β-galactosidase bacterial strain
[0041]Take 4000m of deep sea water, respectively carry out 10 times, 100 times, 1000 times of dilution, take 100 μ L and spread it on the 2216E agar plate containing 1% lactose and X-Gal (40 μ g / mL), place the plate on 4 Cultivate in an incubator for 2 weeks. Pick the blue clones on the plate and streak to obtain a pure culture. Single clones were inoculated into 2216E liquid medium and cultured at 25°C for 2 days. The genomic DNA of the bacterial liquid was extracted, and PCR was performed with 16SrRNA universal primers 27F (AGAGTTTGATCCTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT). After the PCR product was sequenced, the 16SrRNA sequence of the strain was obtained. Blast analysis was performed on the 16SrRNA sequence of strain ML117 and the known 16SrRNA sequence in the NCBI database, and it was found that the β-galactosidase-producing strain ML117 belongs to the genus Alteromonas, and the strain...
Embodiment 2
[0051] Embodiment 2 Construction of low temperature β-galactosidase new gene expression vector
[0052] The single clone of Alteromonas sp.ML117 grown on the 2216E plate was picked and inoculated into the 2216E liquid medium, and cultured at 25°C for 2 days. Take fresh bacterial liquid to extract genomic DNA, and the extracted DNA is passed through primer 1 (CG GAATTC ATGCATACTGTGGCTG) and primer 2 (CCG CTCGAG GGCTACAGGGCAAA) to perform PCR (the underlined parts are restriction enzyme cutting sites EcoR I and Xho I, respectively) to obtain the β-galactosidase gene.
[0053] PCR system (LA Taq enzyme was purchased from TaKaRa)
[0054]
[0055] PCR conditions
[0056] 1.94℃, 5min
[0057] 2.94℃, 1min
[0058] 55℃, 30s
[0059] 72°C, 3min
[0060] Cycle 25 times
[0061] 3.72℃, 5min
[0062] The PCR product was ligated to a T vector for gene sequencing. Sequencing results revealed that the low-temperature β-galactosidase gene derived from Alteromonassp.ML117 has 3...
Embodiment 3
[0064] Embodiment 3 The preparation method of low temperature recombinant β-galactosidase
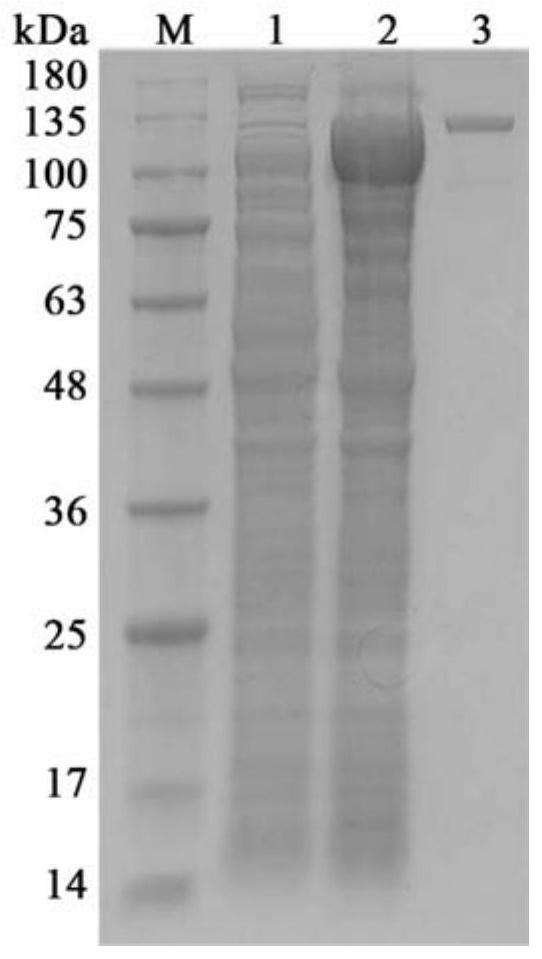
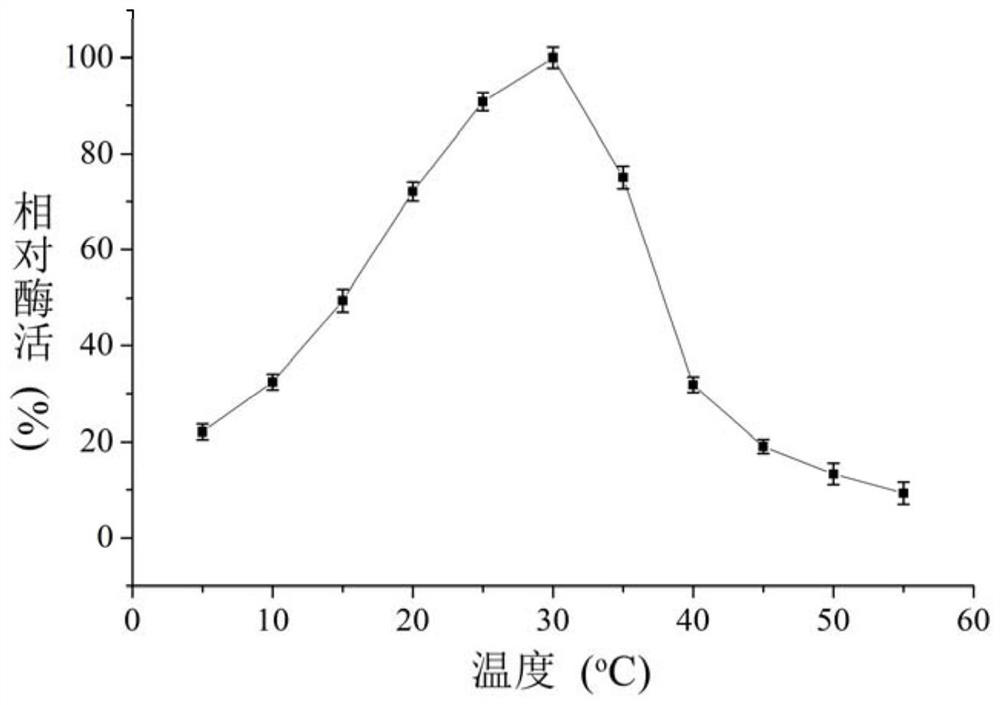
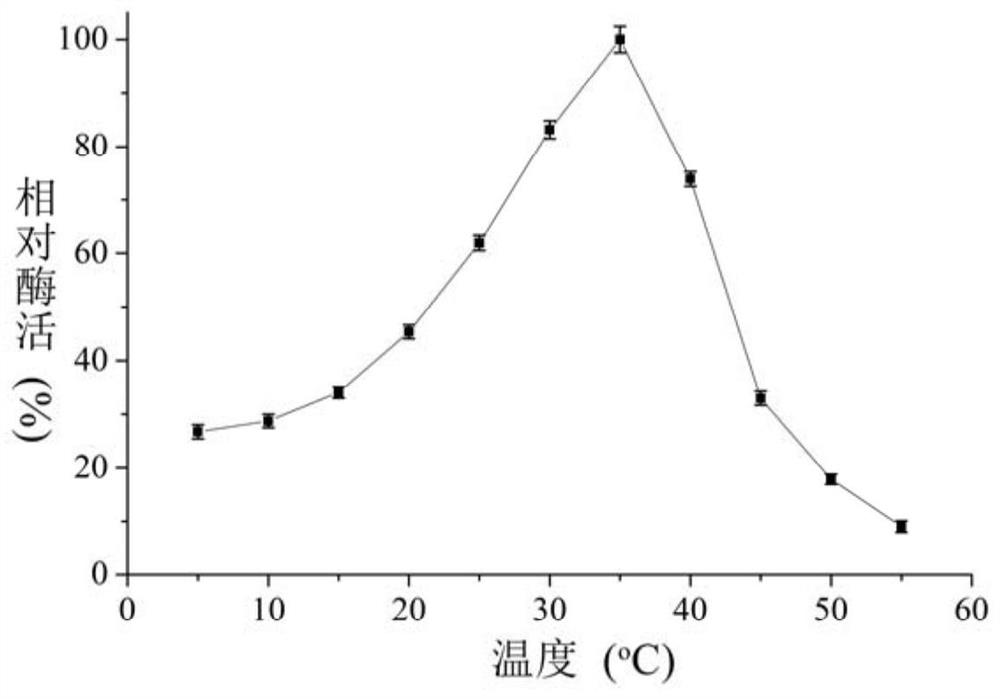
[0065] The above-mentioned recombinant plasmid was transformed into Escherichia coli BL21 (DE3) to obtain a genetically engineered strain of low-temperature β-galactosidase. The strain was inoculated into LB liquid medium containing 50 μg / mL kanamycin and cultured overnight at 37°C. Take 1% culture solution to inoculate and cultivate to OD 600 value 0.6, add IPTG to a final concentration of 0.2mM, and induce at 20°C for 12 hours. The cells were collected by centrifugation at 8500 rpm for 15 minutes at 4°C, and the cells were resuspended with 50 mM phosphate buffer (pH 7.3). The cells were ultrasonically disrupted, and the disrupted solution was centrifuged at 10,000 rpm for 20 minutes at 4°C, and the supernatant was the crude enzyme solution. Wash the affinity chromatography column Ni-NTA Agarose for 5 column volumes with equilibration buffer (50mM phosphate buffer, 500mM NaCl, pH 7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com