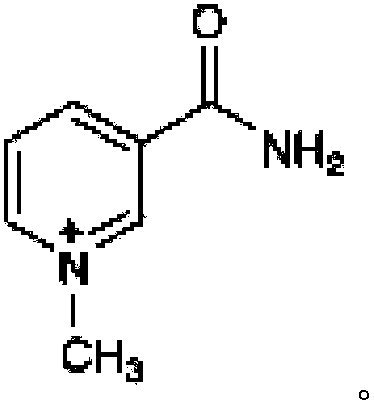
1-methylnicotinamide for the treatment of diseases associated with c-reactive protein
A disease, 1-MNA technology, applied in the direction of resistance to vector-borne diseases, skin diseases, bone diseases, etc., can solve problems such as restrictions on the use of niacin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
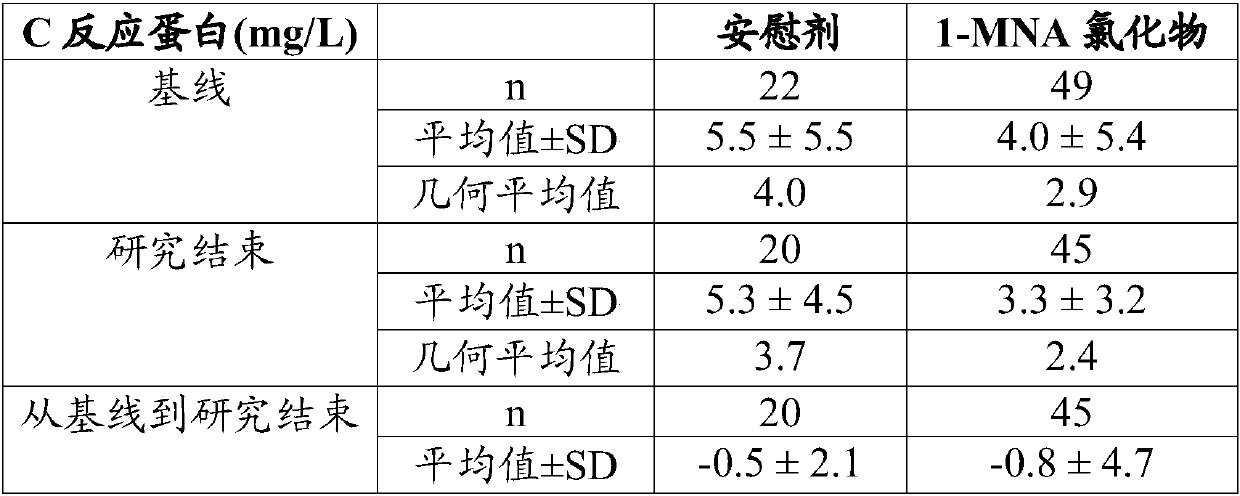
[0096] Treatment with 1-MNA chloride reduces blood or serum CRP levels
[0097] Research design
[0098] The study was a randomized, double-blind, placebo-controlled, mandatory dose-escalation multicenter study.
[0099] standard constrain
[0100] ·At the time of signing the informed consent, the patient is at least 18 years old and ≤80 years old;
[0101] • Women of childbearing potential must have a negative urine pregnancy test at Screening and Visit 4. A woman is considered not of childbearing potential if she meets the following conditions:
[0102] a. Hysterectomy or tubal ligation was performed before the first visit;
[0103] b. Menopause, defined as 12 months without menstruation or FSH level within the menopausal range;
[0104] Women of reproductive potential must agree to use effective birth control throughout the study. Acceptable methods of birth control include: implantable contraceptives, injectable contraceptives, oral contraceptives, transdermal contra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com