Preserving fluid for mesenchymal stem cells, preparation method and application thereof
A technology of quality stem cells and preservation solution, which is applied in the field of stem cell preparation preservation solution, can solve the problems of decreased therapeutic effect and low cell activity, and achieve the effect of avoiding rapid decline in cell activity or even death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
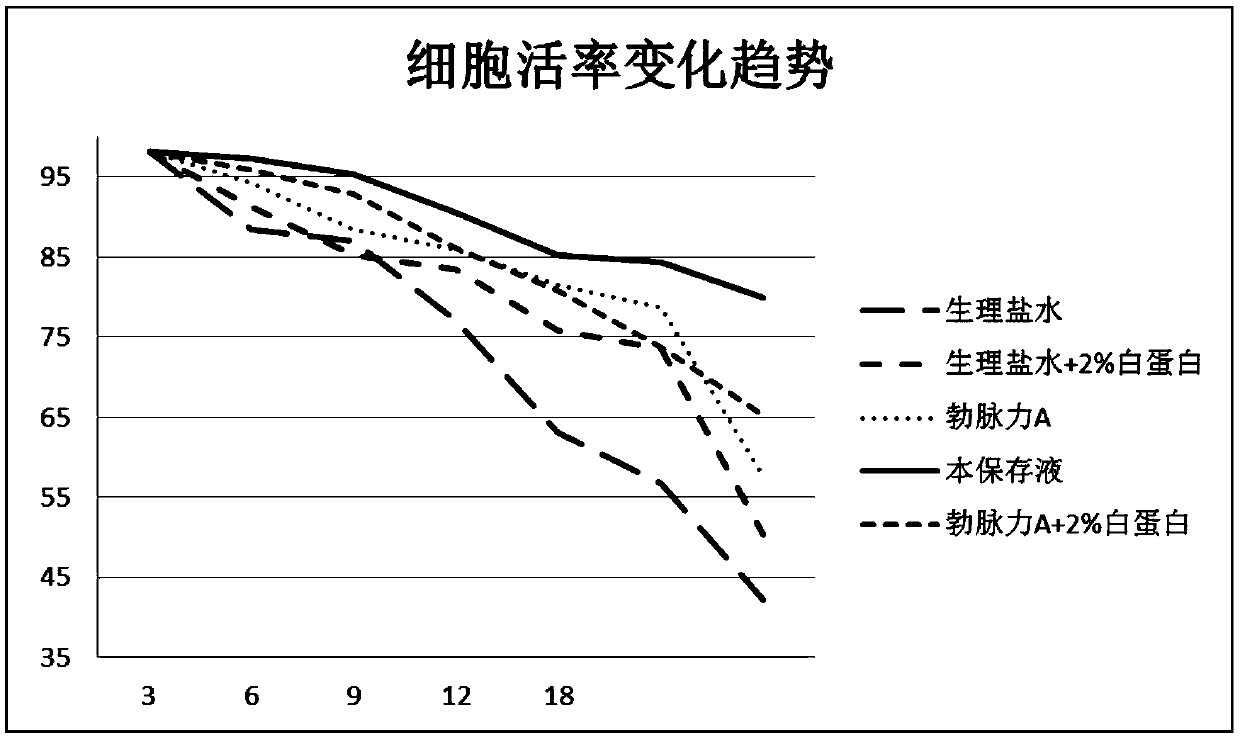
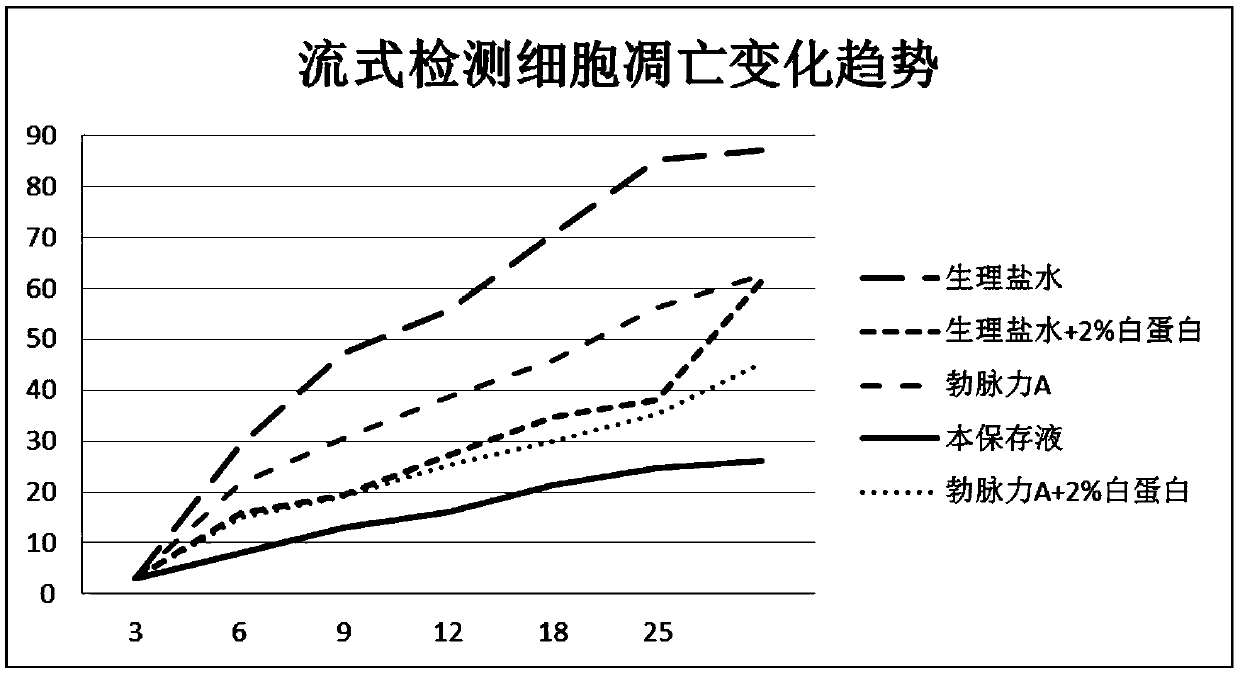
Image
Examples
Embodiment 1
[0044] This embodiment provides a mesenchymal stem cell preservation solution.
[0045] A mesenchymal stem cell preservation solution, which includes Bomaili A electrolyte injection, insulin, estrogen, progesterone, sodium selenite, heparin calcium, human serum albumin, hydrocortisone, adenosine, acetyl cysteine and glutamine.
[0046] In the mesenchymal stem cell preservation solution, the final concentration of insulin is 0.2 μg / L, and the final concentration of estrogen is 1ⅹ10 - 8 mol / L, the final concentration of progesterone is 1ⅹ10 -9 mol / L, the final concentration of sodium selenite is 30nmol / L, the concentration of heparin calcium is 50IU / mL, the final concentration of human albumin is 1%, and the final concentration of hydrocortisone is 1ⅹ10 -6 mol / L, the final concentration of adenosine is 0.5mmol / L, the final concentration of acetylcysteine is 2mmol / L, and the final concentration of glutamine is 30mmol / L.
Embodiment 2
[0048] This embodiment provides a mesenchymal stem cell preservation solution, which is roughly the same as the embodiment, except that the parameters are different, and the differences are as follows:
[0049] A mesenchymal stem cell preservation solution, which includes Bomaili A electrolyte injection, insulin, estrogen, progesterone, sodium selenite, heparin calcium, human serum albumin, hydrocortisone, adenosine, acetyl cysteine and glutamine.
[0050] In the mesenchymal stem cell preservation solution, the final concentration of insulin was 0.18 μg / L, and the final concentration of estrogen was 1.5ⅹ10 -8 mol / L, the final concentration of progesterone is 1ⅹ10 -9 mol / L, the final concentration of sodium selenite is 30nmol / L, the concentration of heparin calcium is 50IU / mL, the final concentration of human albumin is 1%, and the final concentration of hydrocortisone is 1ⅹ10 -6 mol / L, the final concentration of adenosine is 0.5mmol / L, the final concentration of acetylcyst...
Embodiment 3
[0052] This embodiment provides a mesenchymal stem cell preservation solution, which is roughly the same as Embodiments 1-2, the difference lies in the different parameters, and the differences are as follows:
[0053] A mesenchymal stem cell preservation solution, which includes Bomaili A electrolyte injection, insulin, estrogen, progesterone, sodium selenite, heparin calcium, human serum albumin, hydrocortisone, adenosine, acetyl cysteine and glutamine.
[0054] In the mesenchymal stem cell preservation solution, the final concentration of insulin is 0.22 μg / L, and the final concentration of estrogen is 2ⅹ10 -8 mol / L, the final concentration of progesterone is 1ⅹ10 -9 mol / L, the final concentration of sodium selenite is 28nmol / L, the final concentration of heparin calcium is 50IU / mL, the final concentration of human serum albumin is 1%, and the final concentration of hydrocortisone is 1ⅹ10 -6 mol / L, the final concentration of adenosine is 0.5mmol / L, the final concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com