Method of reducing beta-CF3 substituted alpha,beta-unsaturated ketones
A -CF3, unsaturated technology, applied in the α field, can solve the problems of unrealized technology and low reaction efficiency, and achieve the effects of shortening the synthesis process technology route, high reaction yield, and improving synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
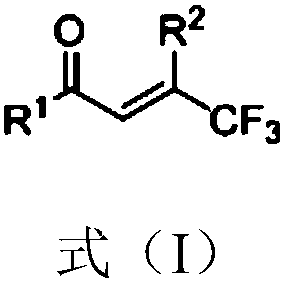
[0026] In a 15mL reaction flask, add 2.0ml of toluene, and then add β-CF shown in the following formula (1) 3 Substituted α,β-unsaturated ketone (69.4mg, 0.25mmol), benzylamine (107μl, 0.75mmol) and metal cobalt perchlorate hexahydrate (2.0mg, 0.005mmol), the molar ratio of the three is 1: 3: 0.005;
[0027] Heat the reaction bottle in an oil bath, stir vigorously at 100°C for 24 hours, stop the reaction, add hydrochloric acid to acidify, and extract with ethyl acetate;
[0028] Get 1mL concentrated and spin-dried and use 1H-NMR to detect the reduced product (beta-CF shown in the following formula (1') 3 substituted saturated ketone), the yield of the reduced product was 89%.
[0029]
[0030] In the above formula (1), (E) represents the E configuration (stereoisomer), which means that the atoms or groups with large atomic numbers connected to the double bond are on different sides of the double bond plane. It is the same in the following examples.
Embodiment 2-10
[0032] The difference between embodiment 2-10 and embodiment 1 is: the β-CF shown in formula (1) 3 The substituted α, β-unsaturated ketone, benzylamine and hexahydrate cobalt perchlorate metal salt have different molar ratios. The rest of the reaction conditions and operations are exactly the same, and details will not be repeated.
[0033] The results of the three molar ratios and the yield of the reduced product in Examples 2-10 are shown in Table 1 below.
[0034] Table 1
[0035]
Embodiment 11
[0037] In a 15mL reaction bottle, add 2.0ml of toluene, and then add β-CF shown in the above formula (1) 3 Substituted α, β-unsaturated ketone (69.4mg, 0.25mmol), benzylamine (160μl, 1.13mmol) and zinc dichloride (0.68mg, 0.005mmol), the molar ratio of the three is 1:4.52:0.02;
[0038] Heat the reaction bottle in an oil bath, stir vigorously at 100°C for 24 hours, stop the reaction, add hydrochloric acid to acidify, and extract with ethyl acetate;
[0039] Get 1mL concentrated and spin-dried and use 1H-NMR to detect the reduced product (β-CF shown in the above formula (1') 3 substituted saturated ketone), the yield of the reduced product was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com