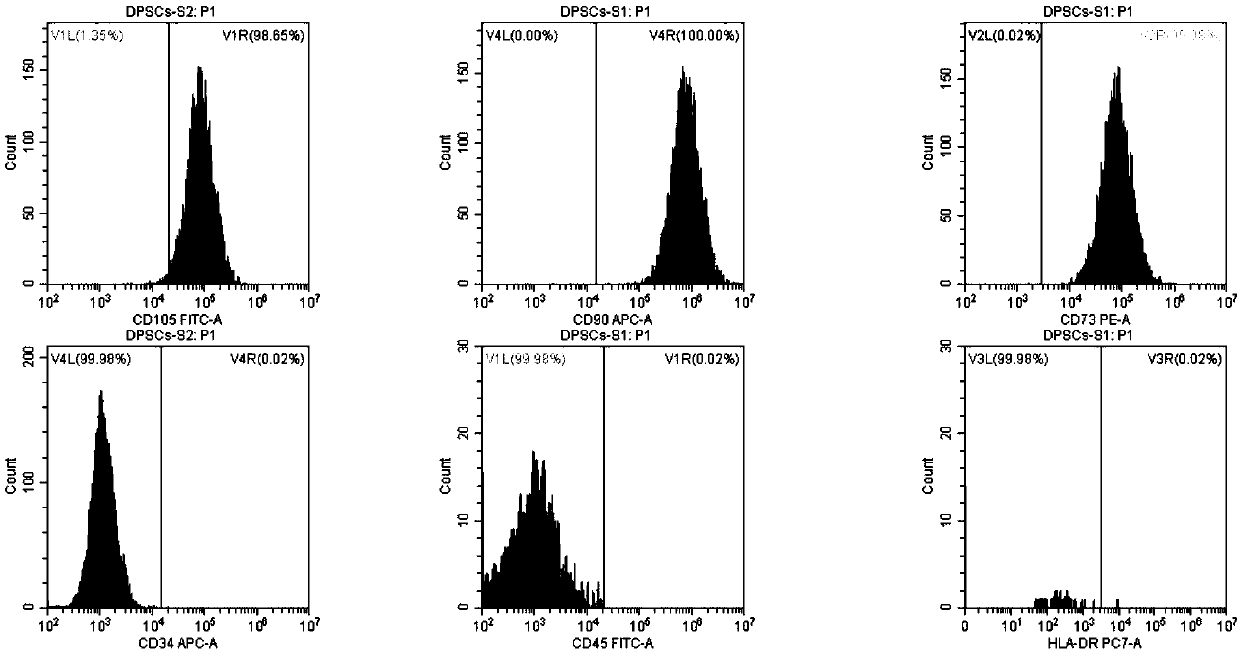
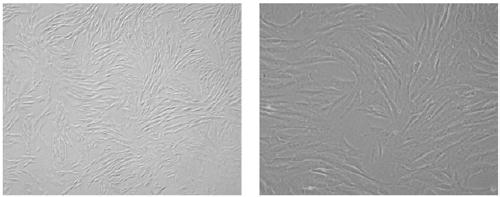
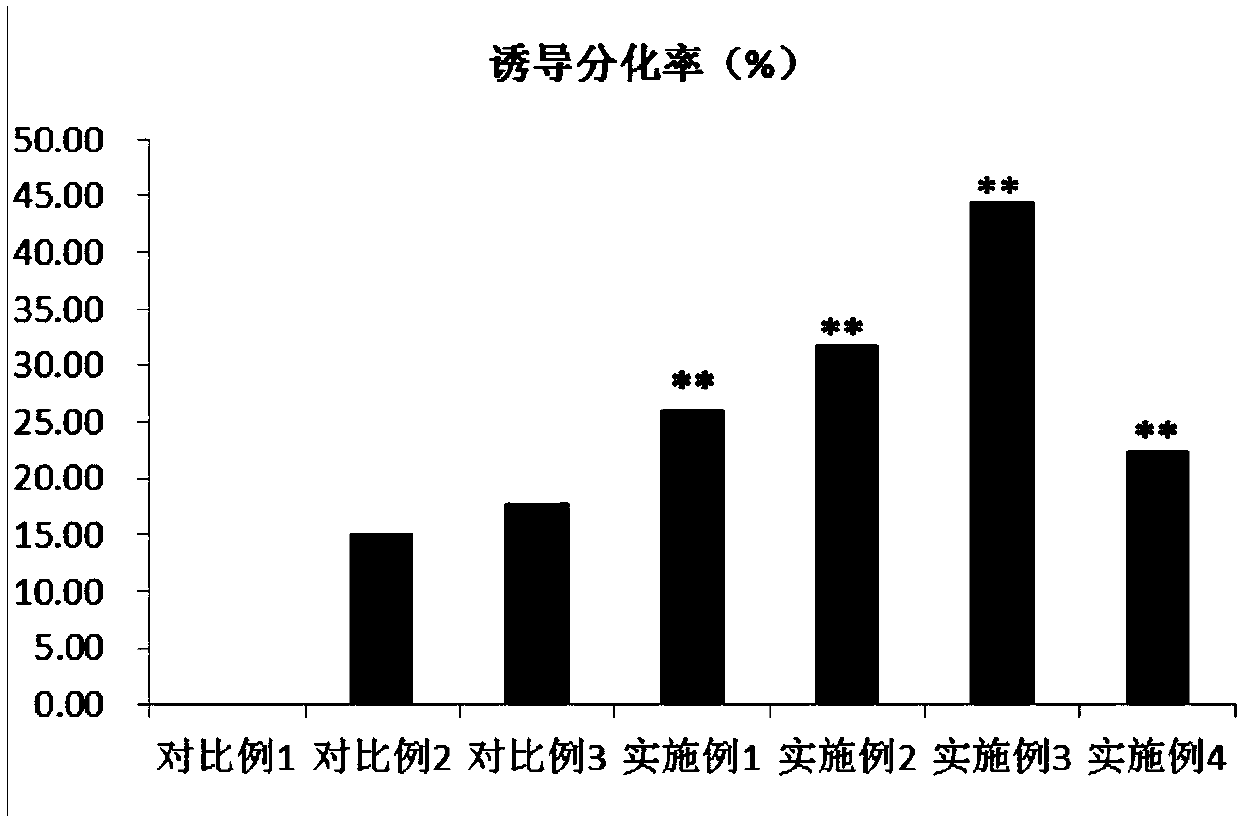
Method for inducing dental pulp stem cells (DPSCs) to be differentiated into cardiomyocyte-like cells
A technique for dental pulp stem cells and cardiomyocyte-like cells, applied in the field of stem cells, can solve problems such as no DPSCs, and achieve the effects of avoiding immune rejection, easy in vitro expansion, and convenient material sampling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Select the 3rd generation DPSCs, press 1×10 4 Cell / mL density was inoculated in a six-well plate with polylysine-treated sterile coverslips, and 2ml of DMEM / F12 medium containing 10% FBS and 10ng / ml EGF was added to each well, and placed at 37°C , 5%CO 2 After 24 hours of cultivation, replace the DMEM / F12 medium containing 10% FBS, 5 μmol / L 5-aza, and 10 μmol / L PFT-α; after 24 hours of induction culture, replace the DMEM / F12 medium containing 15% FBS The F12 medium was maintained for 4 weeks, and new medium was replaced every 2-3 days. After 4 weeks of culture, the cell slides were taken out, and the immunohistochemistry of desmin, troponin I and troponin T was performed according to the instructions of the immunohistochemical staining kit. Staining, DAB color development.
Embodiment 2
[0043] Select the 3rd generation DPSCs, press 1×10 4 Cell / mL density was inoculated in a six-well plate with polylysine-treated sterile coverslips, and 2ml of DMEM / F12 medium containing 10% FBS and 10ng / ml EGF was added to each well, and placed at 37°C , 5%CO 2 After 24 hours of cultivation, replace the DMEM / F12 medium containing 10% FBS, 10 μmol / L 5-aza, and 15 μmol / L PFT-α; after 24 hours of induction culture, replace the DMEM / F12 medium containing 15% FBS The F12 medium was maintained for 4 weeks, and new medium was replaced every 2-3 days. After 4 weeks of culture, the cell slides were taken out, and the immunohistochemistry of desmin, troponin I and troponin T was performed according to the instructions of the immunohistochemical staining kit. Staining, DAB color development.
Embodiment 3
[0045] Select the 3rd generation DPSCs, press 1×10 4 Cell / mL density was inoculated in a six-well plate with polylysine-treated sterile coverslips, and 2ml of DMEM / F12 medium containing 10% FBS and 10ng / ml EGF was added to each well, and placed at 37°C , 5%CO 2 After 24 hours of culture, replace the DMEM / F12 medium containing 10% FBS, 10 μmol / L 5-aza, and 20 μmol / L PFT-α; after 24 hours of induction culture, replace the DMEM / F12 medium containing 15% FBS The F12 medium was maintained for 4 weeks, and new medium was replaced every 2-3 days. After 4 weeks of culture, the cell slides were taken out, and the immunohistochemistry of desmin, troponin I and troponin T was performed according to the instructions of the immunohistochemical staining kit. Staining, DAB color development.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com