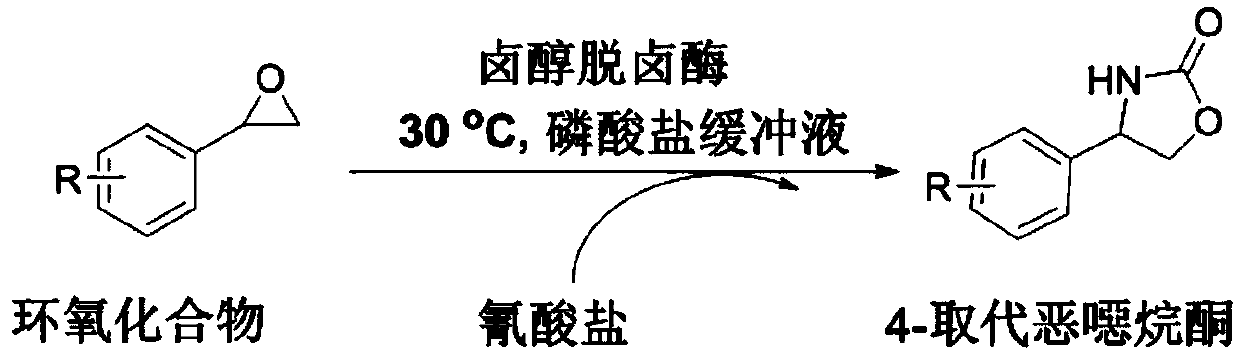
Method of biocatalytically synthesizing 4-substituted oxazolidinone compound
A technology of oxazolidinone and biocatalysis, which is applied in the field of bioengineering to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
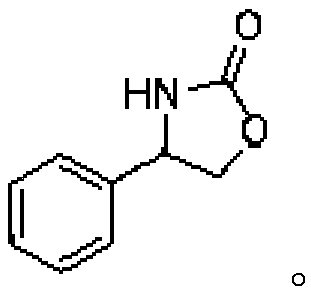
[0024] 4-phenyloxazolidinone synthesis: add 0.45g (dry weight) recombinant halohydrin dehalogenase, 176mg sodium cyanate to 30mL disodium hydrogen phosphate-potassium dihydrogen phosphate (50mM, pH 7.5) buffer solution; 300 μl of dimethyl sulfoxide (as a co-solvent) was dissolved in 108 μl of styrene oxide and added to the buffer. The reaction solution was placed in a temperature-controlled shaker at 30° C., and reacted at 250 rpm for 12 hours. After the reaction was completed, the reaction liquid was extracted with ethyl acetate (2×30 mL), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by silica gel column chromatography (ethyl acetate:petroleum ether=1:1), and reduced Rotary steaming under pressure gave a white solid. 77% yield, 1 HNMR (400MHz, CDCl 3 )δ7.38(m, 5H), 6.21(s, 1H), 4.95(t, J=7.8Hz, 1H), 4.72(t, J=8.7Hz, 1H), 4.17(t, J=7.8Hz, 1H). 13 C NMR (100MHz, CDCl 3 ) δ 160.0, 139.6, 129.3, 128.9, 126.1, 72.7,...
Embodiment 2
[0028] 4-(3-fluorophenyl)-oxan-2-one synthesis: Add 0.45 g (dry weight) of recombinant halohydrin to 30 mL of disodium hydrogen phosphate-potassium dihydrogen phosphate (50 mM, pH 7.5) buffer Halogenase, 176 mg sodium cyanate; 102 microliters of 3-fluorostyrene oxide was dissolved in 300 microliters of dimethyl sulfoxide (as a co-solvent) and added to the buffer. The reaction solution was placed in a temperature-controlled shaker at 30° C., and reacted at 250 rpm for 12 hours. After the reaction was completed, the reaction liquid was extracted with ethyl acetate (2×30 mL), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by silica gel column chromatography (ethyl acetate:petroleum ether=1:1), and reduced Rotary steaming under pressure gave a white solid. 66% yield, 1 H NMR (400MHz, CDCl 3 )δ7.35(dd, J=13.6,7.3Hz,1H),7.10(d,J=7.5Hz,1H),7.03(m,2H),6.89(s,1H),4.96(t,J=7.7 Hz,1H),4.71(t,J=8.7Hz,1H),4.17-4.09(m,1H). 13 C ...
Embodiment 3
[0032]4-(3-Chlorophenyl)-oxan-2-one synthesis: Add 0.45 g (dry weight) of recombinant halohydrin to 30 mL of disodium hydrogen phosphate-potassium dihydrogen phosphate (50 mM, pH 7.5) buffer Halogenase, 176 mg sodium cyanate; 108 microliters of 3-chlorostyrene oxide was dissolved in 300 microliters of dimethyl sulfoxide (as a co-solvent) and added to the buffer. The reaction solution was placed in a temperature-controlled shaker at 30° C., and reacted at 250 rpm for 12 hours. After the reaction was completed, the reaction liquid was extracted with ethyl acetate (2×30 mL), the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by silica gel column chromatography (ethyl acetate:petroleum ether=1:1), and reduced Rotary steaming under pressure gave a white solid. 65% yield, 1 H NMR (400MHz, CDCl 3 )δ7.32(m, 3H), 7.24-7.15(m, 1H), 6.83(s, 1H), 4.99-4.88(t, J=7.8Hz, 1H), 4.70(t, J=8.7Hz, 1H ), 4.12 (dd, J=8.3, 7.0Hz, 1H). 13 C N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com