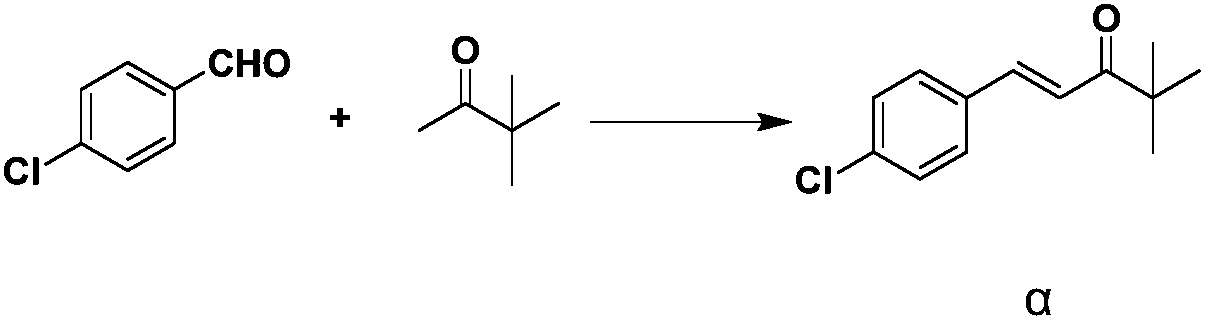
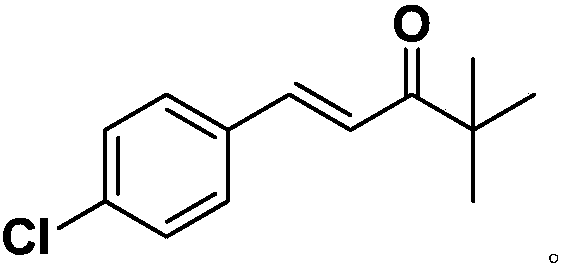
Industrial synthesis method of tebuconazole intermediate alpha
An intermediate, tebuconazole technology, applied in the field of industrial synthesis of tebuconazole intermediate α, can solve the problems of large amount of disproportionated impurities, increased content of target products, waste of raw materials, etc., and achieve mild reaction conditions and low production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] In a preferred embodiment, the industrial synthesis method of the tebuconazole intermediate α comprises the following steps:
[0031] After adding organic solvent, alkali, pinacolone and antioxidant to the reaction vessel, add p-chlorobenzaldehyde in 6-8 batches, and react completely at 30-50°C; cool the reaction solution below 10°C, stir, Suction filtration, take the filter cake and wash it with methanol, and apply the obtained mother liquor to the organic solvent of the next batch of reactions; dry the washed filter cake to obtain the target product tebuconazole intermediate α.
[0032] In a further preferred embodiment, the antioxidant is selected from any one of the following: butylhydroxyanisole, dibutylhydroxytoluene, tert-butylhydroquinone, and sodium dithionite.
[0033] In a further preferred embodiment, the molar weight of the antioxidant is 0.1-5.0% of the molar weight of p-chlorobenzaldehyde.
[0034] In a further preferred embodiment, the molar ratio of th...
Embodiment 1
[0040] Add 120 mL of methanol and 2.5 g of sodium hydroxide (0.0625 mol, 0.2 eq) into a 250 mL three-necked flask, and stir mechanically to cool down to room temperature. Next, add 31.2g (0.313mol, 1.1eq) of pinacolone, then add 0.26g (1.43mmol, 0.5%eq) of butylated hydroxyanisole, raise the temperature to 43°C, and add 5.0g of p-chlorobenzaldehyde every 0.5h , A total of 8 batches were added, with a total of 40.0g (0.286mol, 1.0eq); the addition was completed and the reaction was held for 12 hours, and solids were precipitated during the reaction. After the reaction is complete, cool the reaction liquid to below 10°C, stir for 2 hours, filter with suction, take the filter cake and rinse it with 10mL of methanol, dry the washed filter cake to obtain the tebuconazole intermediate α; the mother liquor is reserved for the next batch The reaction is applied mechanically, specifically, the mother liquor is applied mechanically 3 times, and the pinacolone add-on is 31.2g (0.313mol, ...
Embodiment 2
[0043]Add 120 mL of methanol and 3.0 g of sodium hydroxide (0.075 mol, 0.26 eq) into a 250 mL three-necked flask, and stir mechanically to cool down to room temperature. Next, add 31.2g (0.313mol, 1.1eq) of pinacolone, then add 0.10g (0.57mmol, 0.2%eq) of butylated hydroxyanisole, raise the temperature to 45°C, and add 6.67g of p-chlorobenzaldehyde every 0.5h , a total of 6 batches were added, with a total of 40.0g (0.286mol, 1.0eq); the addition was completed and the reaction was kept for 15 hours, and solids were precipitated during the reaction. After the reaction is complete, cool the reaction liquid to below 10°C, stir for 2 hours, filter with suction, take the filter cake and rinse it with 10mL of methanol, dry the washed filter cake to obtain the tebuconazole intermediate α; the mother liquor is reserved for the next batch The reaction is applied mechanically, specifically, the mother liquor is applied mechanically 1 time, and the pinacolone addition is 30g (0.3mol, 1.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com