Method for preparing general type CAR-T cells by using CRISPR/Cas9+AAV
A general-purpose, cell-based technology, applied in the field of preparation of general-purpose CAR-T cells, can solve problems such as limited expansion capacity, inability to obtain cells, and inability to adopt quality, so as to improve safety and controllability, promote application, and reduce Effect of treatment time cost and financial cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Universal CD19 CAR-T
[0028] (1) Preparation process of general-purpose CD19 CAR-T:
[0029] Separation and purification of CD3+ T cells → static culture → activation → CRISPR / Cas gene editing → adeno-associated virus infection of T cells → magnetic bead sorting to remove TCRαβ-positive cells → aliquot storage.
[0030] (2) Selection of functional region gene sequences: KOZAC, scFv CD19 fmc63 (19), CD8A, CD28, CD137 (4-1BB), CD247 (CD3-zeta ) functional region coding sequence.
[0031] (3) Whole-gene synthesis of the CAR gene sequence, including: BstXⅠ restriction site + TRAC left homology arm + KOZAC + CD19 monoclonal antibody fmc63 (19) single-chain variable region + hinge + transmembrane region CD8a + co-stimulatory Molecule CD137 (4-1BB) + intracellular signal transduction region CD247 (CD3-zeta) + stop codon + TRAC right homology arm + BamHI restriction site.
[0032] (4) Insert the vector pAAV-MCS through the restriction site to comple...
Embodiment 2
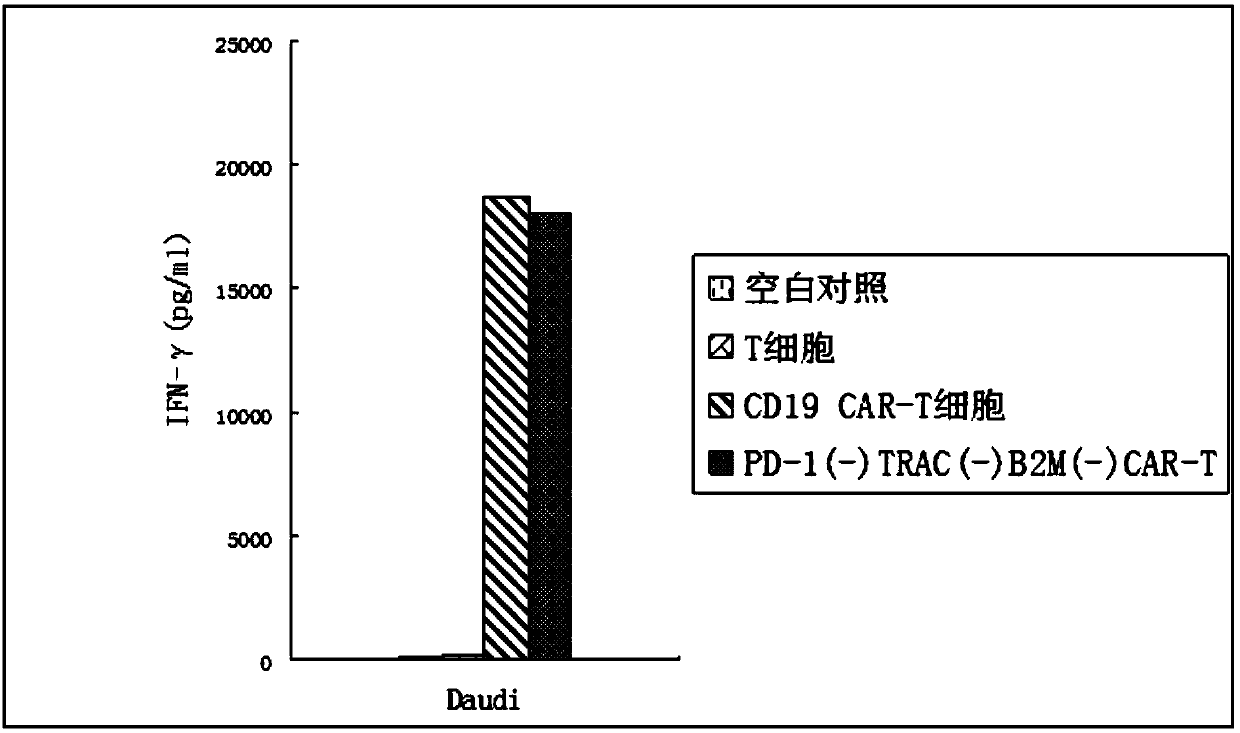
[0045] Embodiment 2 Quality Inspection
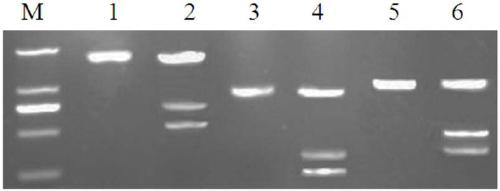
[0046] (1) Mismatch enzyme method (Surveyor method) detects CRISPR / Cas9 activity or mutation efficiency, and evaluates the success rate of gene editing.
[0047] DNA extraction and PCR amplification: Extract gene-edited T cell DNA and perform PCR amplification, using PD-1, TRAC, and B2M gRNA as primers respectively,
[0048]
[0049] Amplification conditions: pre-denaturation at 94°C for 10 minutes, denaturation at 94°C for 30 seconds, annealing at 63°C for 20 seconds, extension at 72°C for 1 minute, amplification for 3 to 5 cycles, and final extension at 72°C for 7 minutes. The PCR product was stored at 4°C for later use.
[0050] DNA hybridization: The unedited T cell DNA amplification product is mixed with the amplification product of the sample to be tested in equal volumes, denatured at 95°C for 5 minutes, and then gradually lowered to 25°C at a rate of 0.1°C every 4 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com