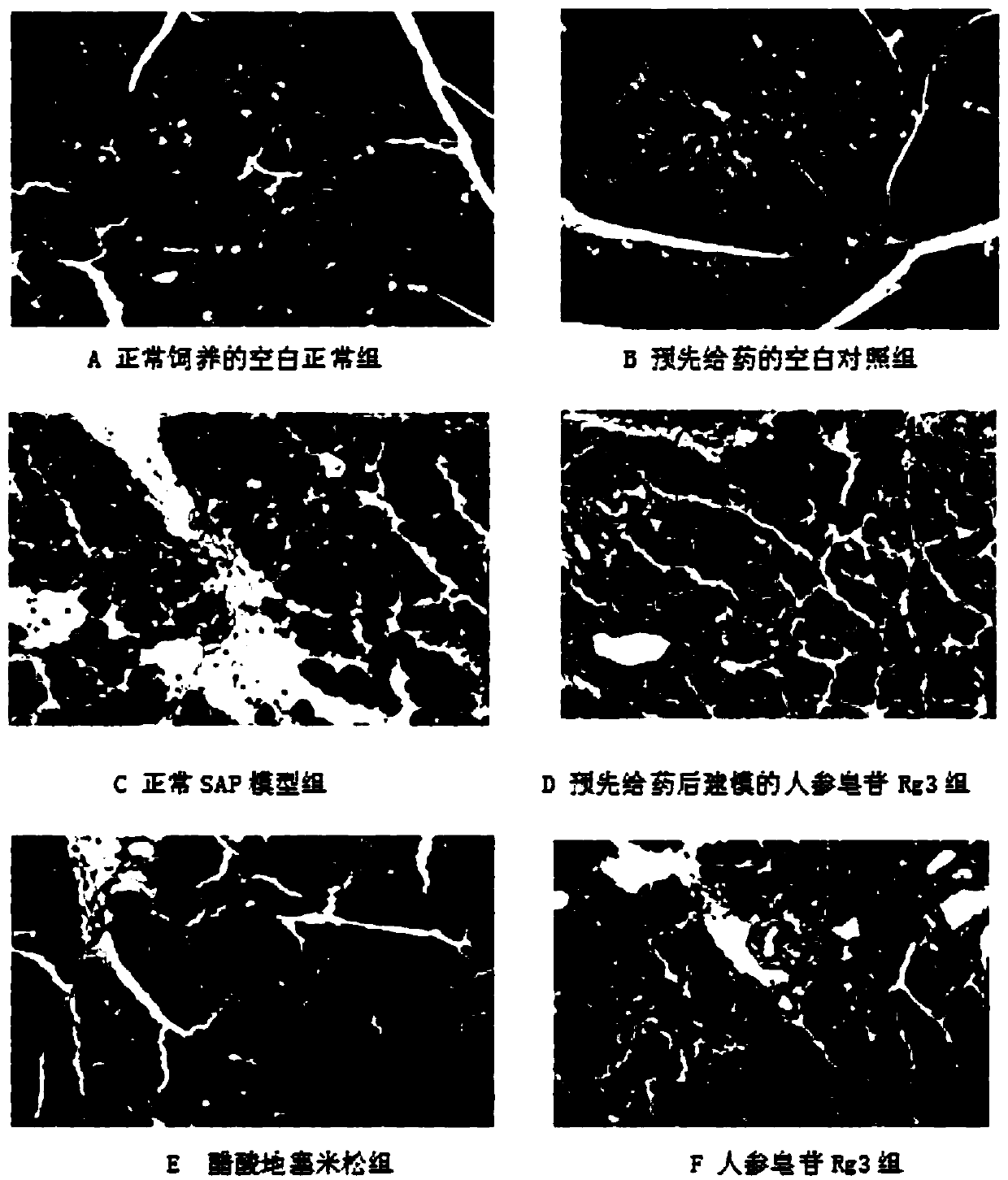
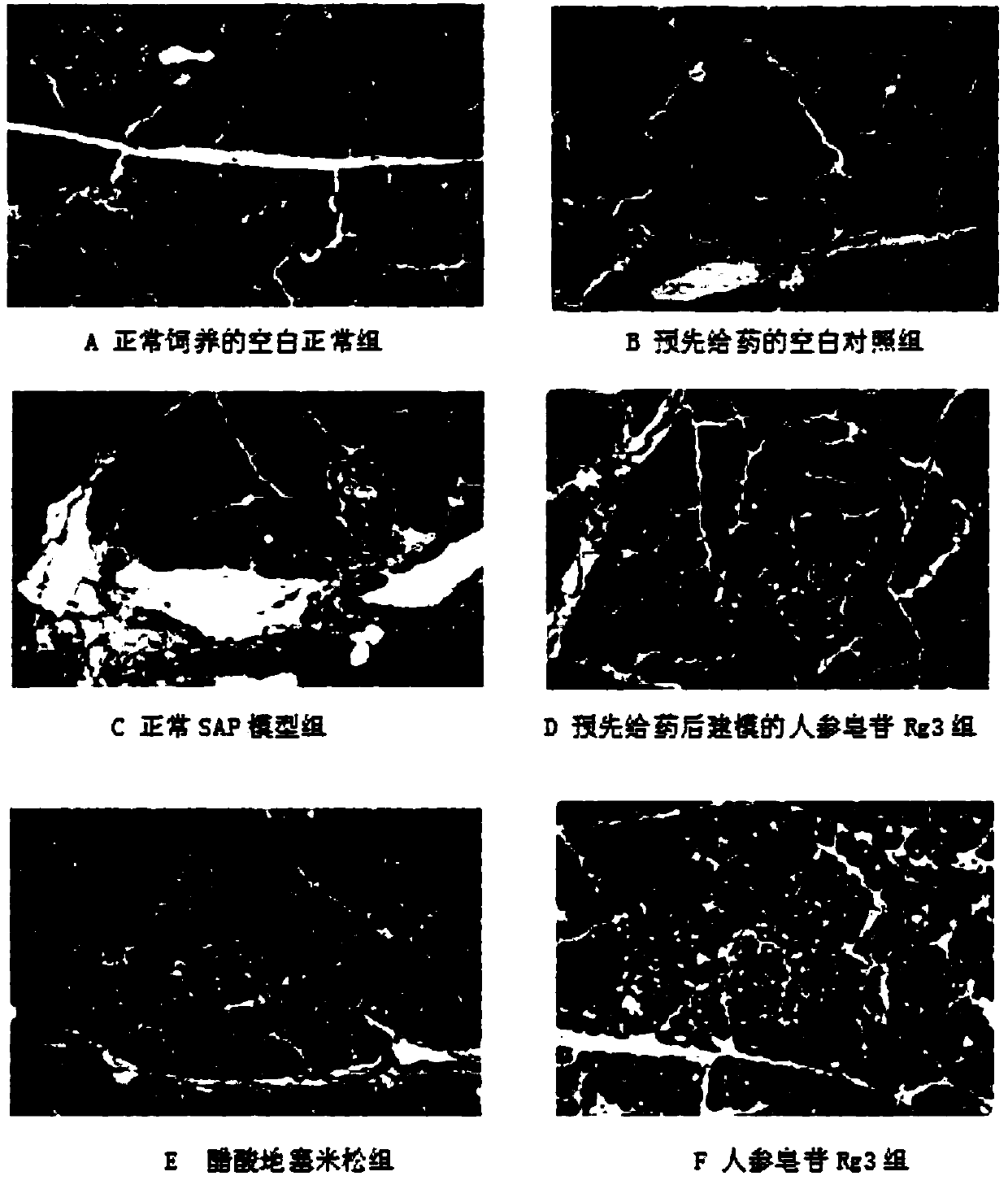
Application of ginsenoside Rg3 in preparing drug for preventing and/or treating severe acute pancreatitis
A technology of acute pancreatitis and ginsenosides, applied in the directions of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the problems such as no reports on the treatment of severe acute pancreatitis, achieve significant anti-pancreatitis, reduce positive expression, improve pathological effect of phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention has no special limitation on the preparation method of the tablet, and the conventional tablet preparation method in the art can be used. During the specific implementation of the present invention, the tablet is preferably prepared by the following method:
[0024] 1) Mix ginsenoside Rg3, powdered sugar, lactose and sodium carboxymethyl starch, and sieve to obtain a fine powder;
[0025] 2) mixing the fine powder described in step 1) with a 3% PVPK30 aqueous solution by weight,
[0026] Perform granulation, drying and sizing to obtain granules;
[0027] 3) The granules described in step 2) are mixed with magnesium stearate, and compressed into tablets to obtain tablets.
[0028] In the present invention, firstly, ginsenoside Rg3, powdered sugar, lactose and sodium carboxymethyl starch are mixed and sieved to obtain fine powder; the sieve mesh aperture is preferably 80-120 mesh, more preferably 100 mesh.
[0029] After the fine powder is obtained...
Embodiment 1
[0050] The preparation of embodiment 1 ginsenoside Rg3 sheet
[0051] prescription:
[0052]
[0053]
[0054] Preparation method: Weigh the prescribed amount of ginsenoside Rg3, powdered sugar, lactose and sodium carboxymethyl starch, mix well and pass through a 100-mesh sieve, add 3% PVPk30 aqueous solution to make a soft material, granulate with a 20-mesh sieve, and heat at 60°C Dry for 3 hours, sieve with 18 meshes, add the prescribed amount of magnesium stearate, mix evenly, punch tablets with dimples, adjust the tablet weight to 150 mg, and obtain the product.
Embodiment 2
[0055] The preparation of embodiment 2 ginsenoside Rg3 dropping pills
[0056] prescription:
[0057]
[0058] Preparation method: Weigh the prescribed amount of PEG400, PEG4000, sodium carboxymethyl starch, and povidone K30, add them to the dripping pill machine to melt at 95°C, add the prescribed amount of ginsenoside Rg3 while stirring, mix well, and the temperature of the drug material is 85°C, medicine material with drop distance of 20cm, press 28 drops.min -1 Drop into liquid paraffin at a specified rate, condense at 10°C, solidify, and form drop pills, then take them out and dry them to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com