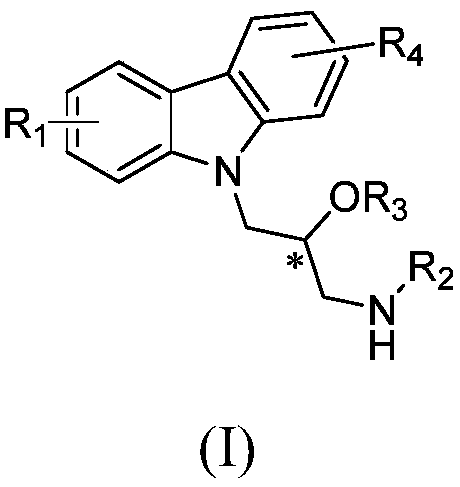
Preparation method for carbazolylv isopropanolamine derivative with chiral center and application
A carbazolyl isopropanolamine, chiral technology, applied in the field of carbazolyl isopropanolamine compounds and their preparation, can solve problems affecting citrus yield, citrus rot, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of intermediate (R)-3,6-di-tert-butyl-9-(2-methyloxyethylene)-9H-carbazole
[0058] Put 3,6-di-tert-butylcarbazole (29.9mmol) and potassium hydroxide (35.9mmol) in a 100mL round bottom flask, add 30mL DMF to it and stir, add R-epichlorohydrin (35.9mmol ) after 8 hours of reaction, stop the reaction, extract, and column chromatography (eluent PE:EA=30:1), to obtain a white solid with a yield of 54.5%.
Embodiment 2
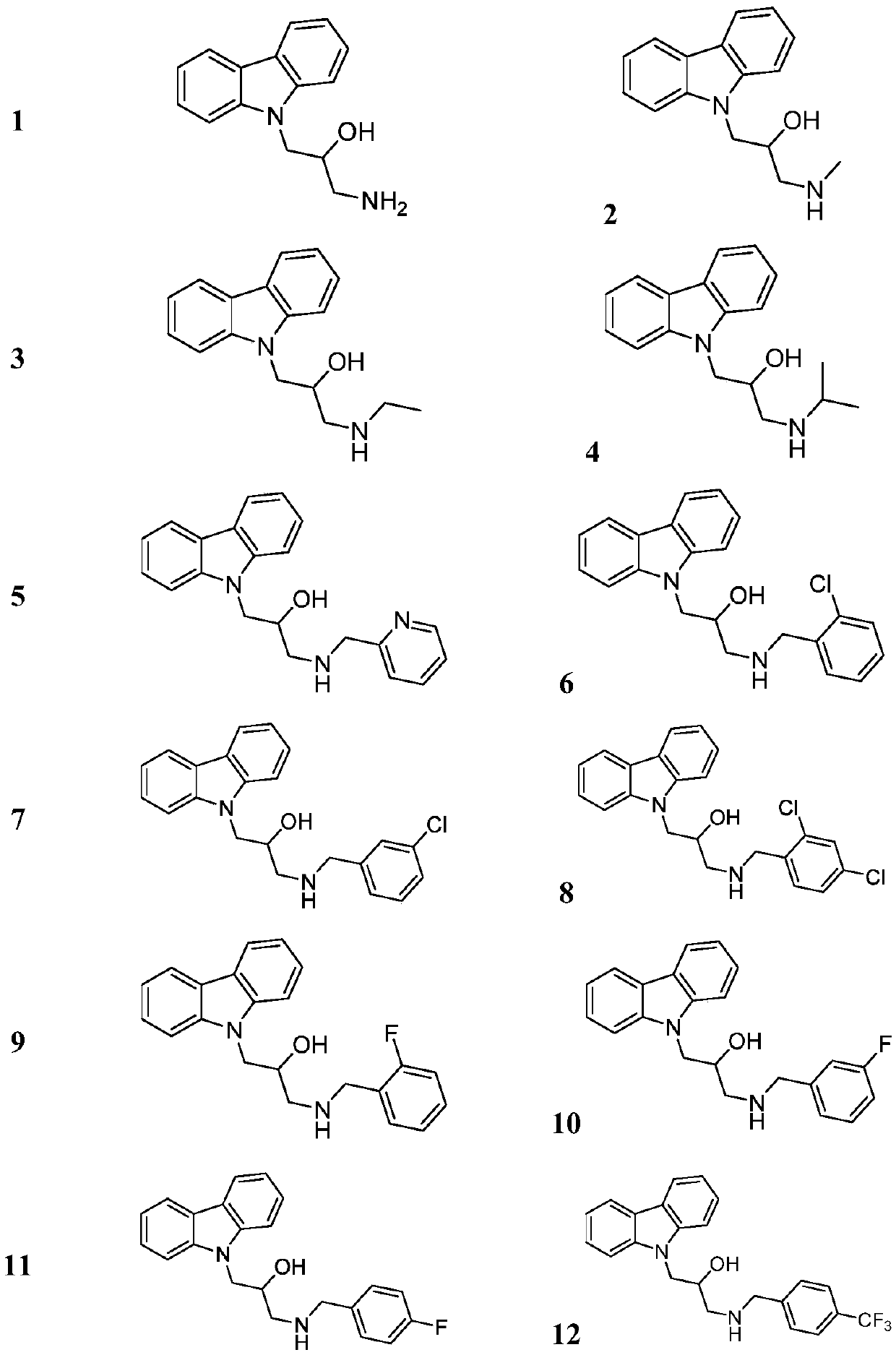
[0059] Embodiment 2: Preparation of target compound 1-(9H-carbazolyl-9-yl)-3-((2-chlorobenzyl)amino)-2-hydroxypropanol
[0060] Add 9-(2-methyloxyethylene)-9H-carbazole (0.90mmol) and potassium carbonate (0.90mmol) to 2-chlorobenzylamine (1.80mmol) dissolved in 5mL isopropanol solution, 60°C After reacting for 6 hours, the reaction was stopped, solvent removal, column chromatography (eluent CH 2 Cl 2 :CH 3 OH=200:1) to obtain a white solid with a yield of 74.7%.
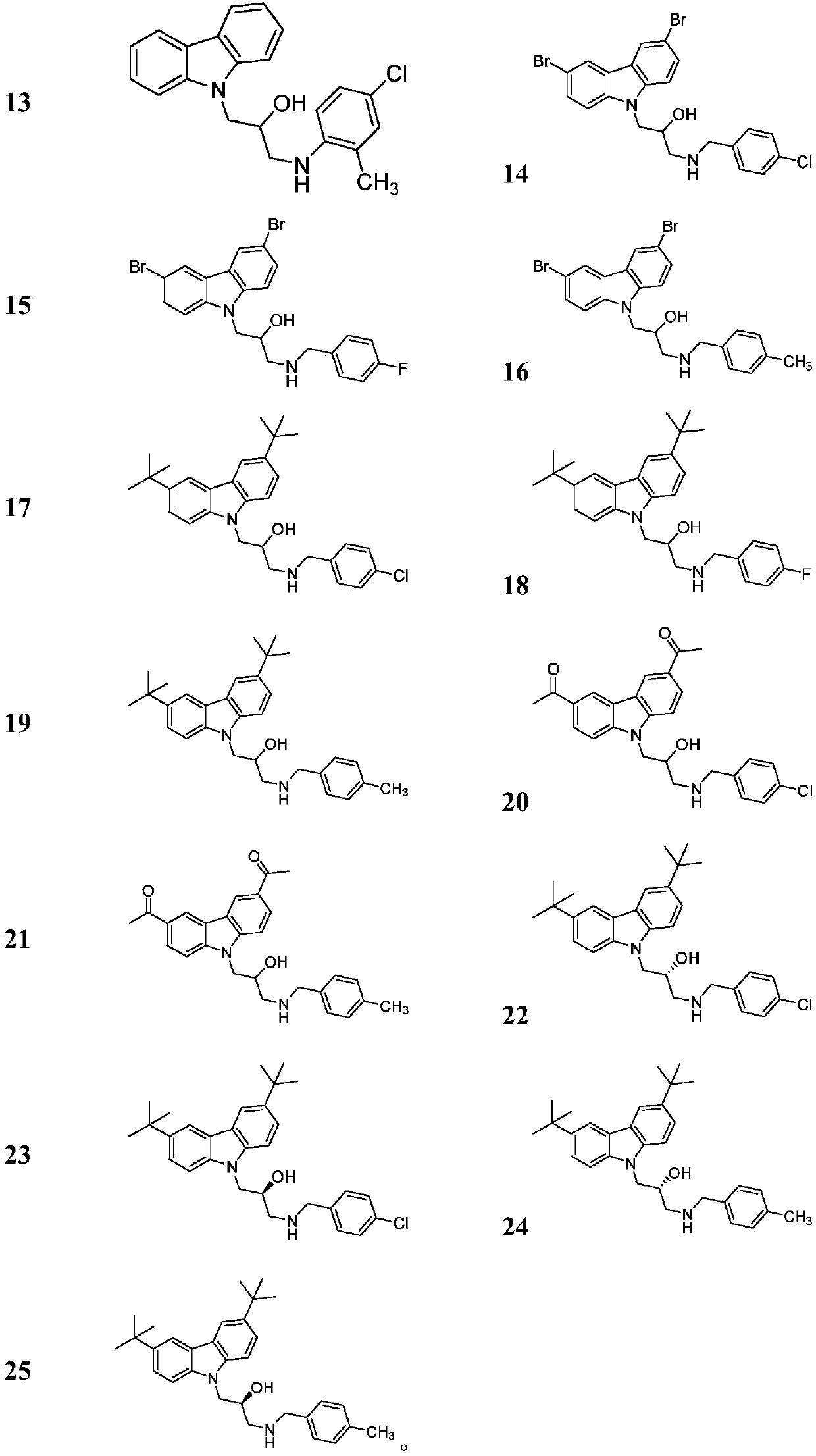
[0061] Other compounds were prepared using corresponding starting materials in a similar manner to Example 1 / 2. The structure, H-NMR and C-NMR data of the synthesized carbazolyl isopropanolamine structure compounds are shown in Table 1, and their physical and chemical properties are shown in Table 2.
[0062] H NMR spectrum and carbon spectrum data of some compounds in Table 1
[0063]
[0064]
[0065]
[0066]
[0067]
[0068]
[0069]
[0070] Table 2 Physicochemical properties of some t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com