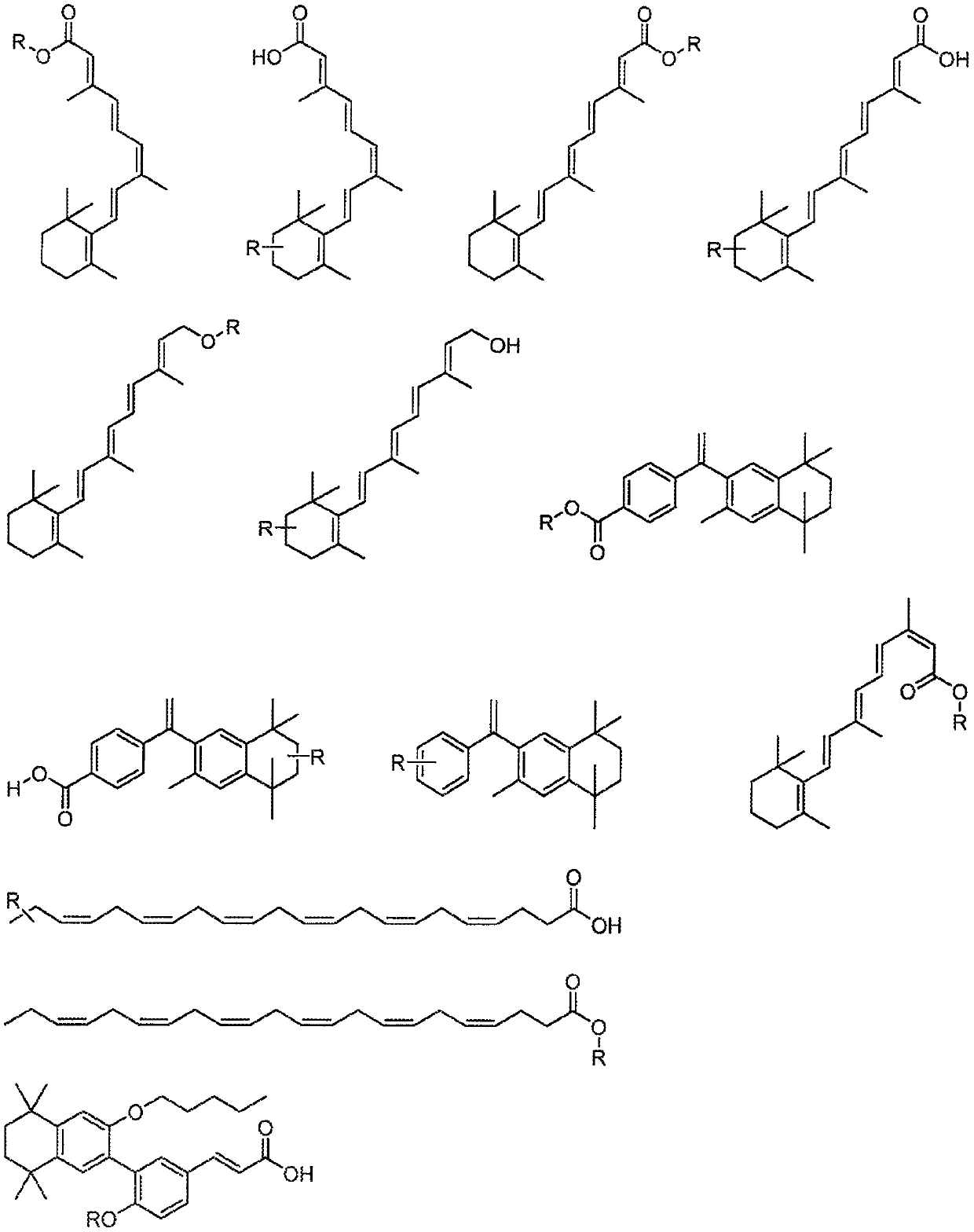
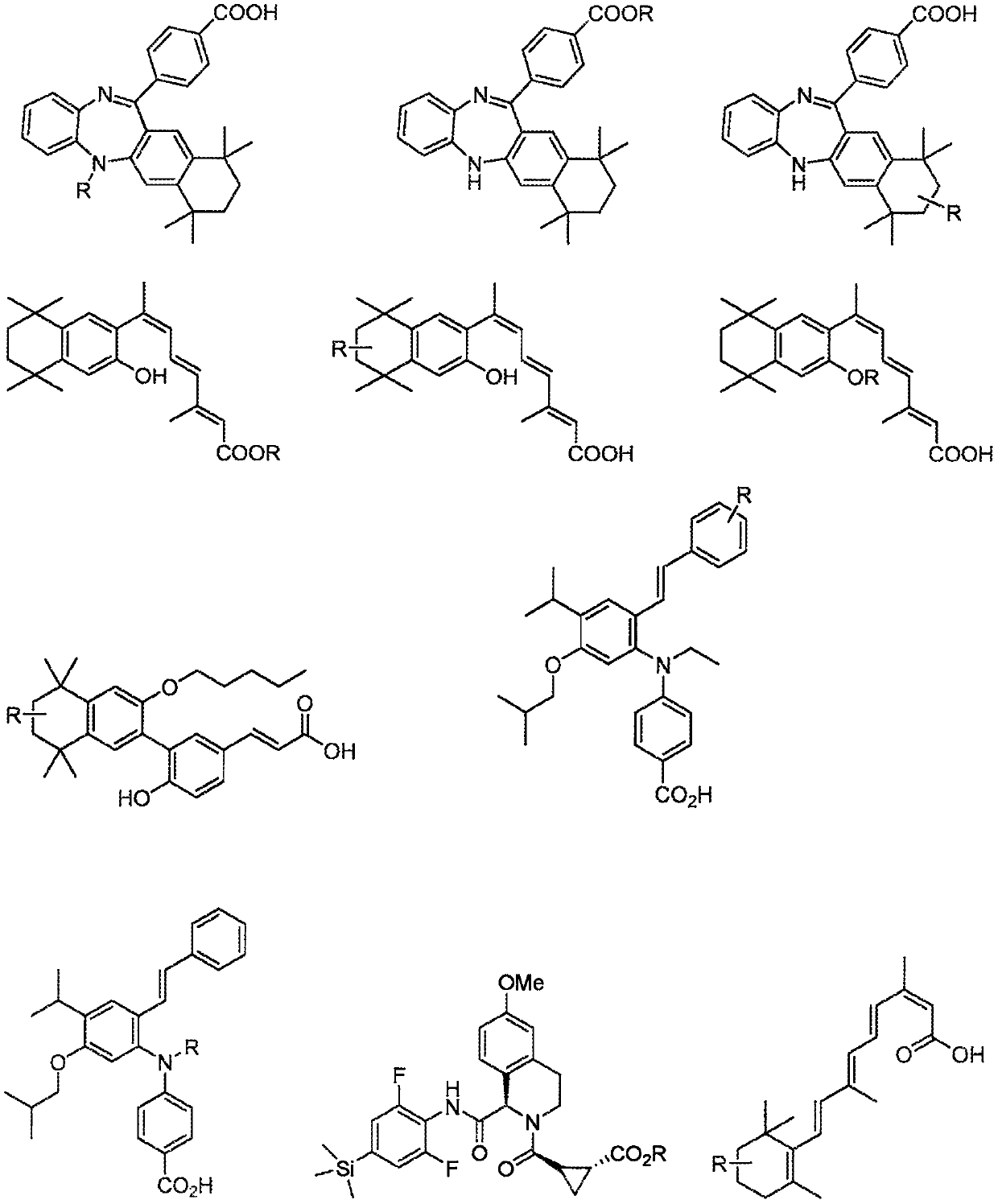
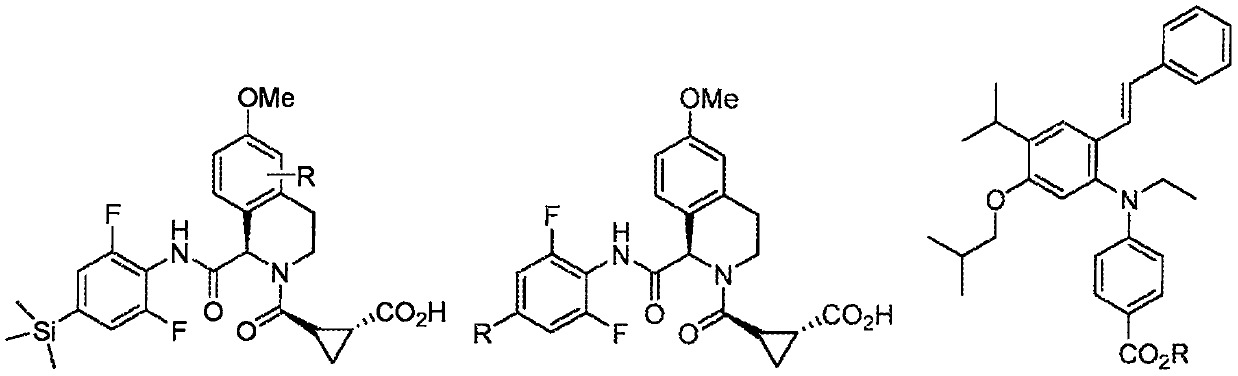
C3-carbon linked glutarimide degronimers for target protein degradation
A determinant, ligand-targeting technology applied in the field of C3-carbon-linked glutarimide degronomers for target protein degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0961] Example 1: 1,3-(4-bromophenyl)piperidine-2,6-dione
[0962]
[0963] Dimethyl 2-(4-bromophenyl)glutarate
[0964]
[0965] Sodium hydride (1.1 equiv) was suspended in THF and cooled to 0 °C. Methyl 2-(4-bromophenyl)acetate (1 equiv) was added dropwise and the reaction was mixed for 1 hour. Methyl 3-bromopropionate (1 eq.) was added dropwise. When the reaction was judged complete, it was quenched with aqueous ammonium chloride and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, concentrated and purified by silica gel chromatography to provide dimethyl 2-(4-bromophenyl)glutarate. (Eur JOC, 2015, (3), 556)
[0966] 3-(4-Bromophenyl)piperidine-2,6-dione
[0967]
[0968] To a stirred solution of sodium amide prepared in situ in liquid ammonia from metallic sodium and ammonia in the presence of a catalytic amount of iron(III) chloride was added 2-(4-bromophenyl)glutaric acid at -33 °C A solution of dimethyl ester in te...
Embodiment 2
[0969] Example 2: 3-(4-bromophenyl)piperidine-2,6-dione-3-d
[0970]
[0971] tert-butyl 3-(4-bromophenyl)-2,6-dioxopiperidine-1-carboxylate
[0972]
[0973] Catalytic amounts of DMAP and di-tert-butyl dicarbonate (1.05 equiv) were added to a solution of 3-(4-bromophenyl)piperidine-2,6-dione in acetonitrile at ambient temperature. Once the reaction was complete, the volatiles were removed by rotary evaporation and the residue was purified by silica gel chromatography to afford tert-butyl 3-(4-bromophenyl)-2,6-dioxopiperidine-1-carboxylate.
[0974] 3-(4-Bromophenyl)-2,6-dioxopiperidine-1-carboxylic acid tert-butyl ester-3-d
[0975]
[0976]A solution of tert-butyl 3-(4-bromophenyl)-2,6-dioxopiperidine-1-carboxylate in anhydrous THF was treated with bis(trimethylsilyl)amino Lithium (1.1 equivalents) was treated for 1 hour. The reaction was quenched with deuterated acetic acid (Bioorg. Med. Chem. Lett. 2003, 13, 3415) and warmed to ambient temperature. The crude r...
Embodiment 3
[0980] Example 3: 3-(4-bromophenyl)-3-fluoropiperidine-2,6-dione
[0981]
[0982] tert-butyl 3-(4-bromophenyl)-3-fluoro-2,6-dioxopiperidine-1-carboxylate
[0983]
[0984] A solution of tert-butyl 3-(4-bromophenyl)-2,6-dioxopiperidine-1-carboxylate in anhydrous THF was treated with bis(trimethylsilyl)amino Lithium (1.1 equivalents) was treated for 1 hour. A minimal amount of N-fluorobenzenesulfonimide (Bioorg. Med. Chem. Lett. 2003, 13, 3415) in anhydrous THF was added and the reaction was warmed to ambient temperature then quenched. The crude reaction was extracted with ethyl acetate and the combined organic layers were dried over sodium sulfate, concentrated and purified by silica gel chromatography to provide 3-(4-bromophenyl)-3-fluoro-2,6-dioxopiper tert-Butyl pyridine-1-carboxylate.
[0985] 3-(4-Bromophenyl)-3-fluoropiperidine-2,6-dione
[0986]
[0987] A solution of tert-butyl 3-(4-bromophenyl)-3-fluoro-2,6-dioxopiperidine-1-carboxylate in DCM was treated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com