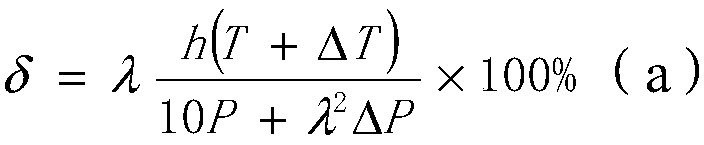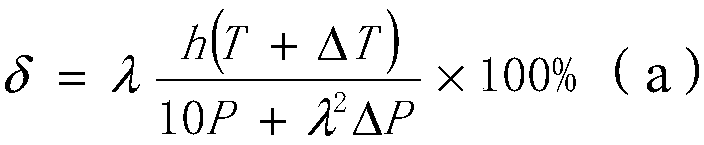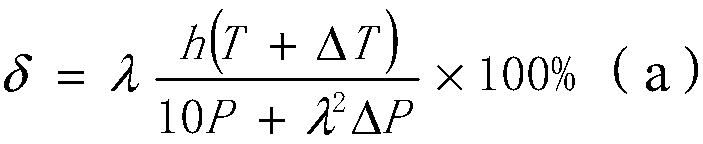Method for preparing p-phenylenediamine
A technology of p-phenylenediamine and p-nitroaniline, which is applied in the field of preparation of p-phenylenediamine, can solve the problems of low purity of p-phenylenediamine, excessive industrial wastewater, and environmental pollution, and achieve simple and safe production process with less pollution , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of p-phenylenediamine, specifically comprising the following steps:
[0026] S1: Take a certain amount of p-nitroaniline, hydrogen, deionized water and a new type of hydrogenation nickel catalyst by weight, in which water is used as a solvent, hydrogen participates in the reaction and is excessive, and p-nitroaniline is ground by a grinder in advance, and the p-nitroaniline The mass ratio of nitroaniline to water is 1:3, the new nickel hydrogenation catalyst accounts for 2.6% of the mass of p-nitroaniline, and the mass ratio of p-nitroaniline to hydrogen is not more than 1 / 3. Nitroaniline takes 1.38kg, wherein in this application, the relative molecular mass of p-nitroaniline is 138, and the molecular mass of p-phenylenediamine phase is 108;
[0027] S2: Put p-nitroaniline, water and a new nickel hydrogenation catalyst into the reaction kettle, and replace the air in the reaction kettle with nitrogen. About 1MPa, the number of replacements with nit...
Embodiment 2
[0042] On the basis of embodiment 1, other parameters are constant, change the reaction time of step S3, i.e. the value of h, wherein h takes the value of 3 hours, brings in the formula (a), can get δ greater than 1, then proves that to nitric acid The conversion rate of phenylenediamine reaches 100%, and the yield of p-phenylenediamine is 1.069kg, then the yield is 1.069 / 1.08=98.98%, and by gas chromatograph analysis, the purity of p-phenylenediamine exceeds 99.914%; It can be seen that the phase Compared with Example 1, the yield and purity of p-phenylenediamine in Example 2 are slightly improved; the actual conversion rate of p-nitroaniline calculated by gas chromatograph analysis is 100%, which is the same as the calculation result of the formula.
[0043] If the reaction time is lower than 2 hours, it is brought into the formula (a), and the obtained δ is 49.5%. For example, the reaction time is 1 hour, and the yield of p-phenylenediamine is 0.956kg, then the yield is 0.95...
Embodiment 3
[0046] On the basis of Example 1, other parameters remain unchanged, and ΔT is changed, wherein ΔT takes a value of 10°C, that is, the reaction temperature of 60°C in step S3 is increased to 70°C, so that the reaction is carried out at 70°C. Bring in the formula (a), can get δ greater than 1, then prove that the conversion rate of p-nitroaniline reaches 100%, the yield of p-phenylenediamine is 1.071kg, then yield is 1.071 / 1.08=99.16%, by According to gas chromatography analysis, the purity of p-phenylenediamine is 99.916%; the actual conversion rate of p-nitroaniline calculated by gas chromatograph analysis is 100%, which is the same as the formula calculation result.
[0047]If the reaction temperature is lower than 60°C, it is brought into the formula (a), and the obtained δ is less than 1, which proves that the conversion rate of p-nitroaniline decreases. For example, the reaction temperature is 40°C, and the conversion rate is 66% calculated by the formula. The yield of ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com