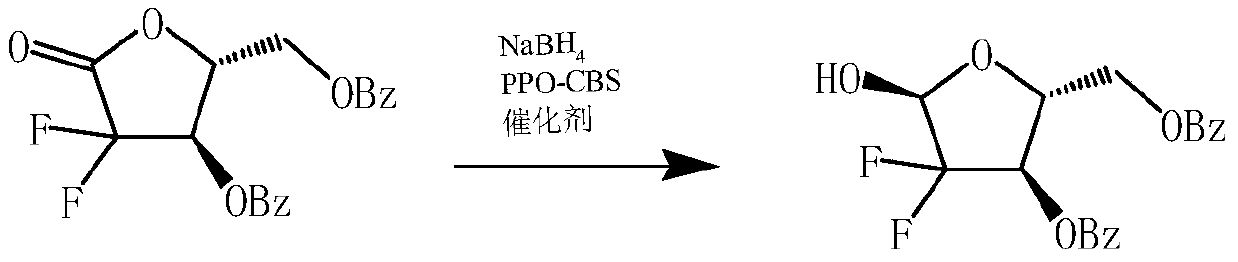
A kind of preparation method of 2-deoxy-2,2-difluoro-d-erythro-pentofuranose-3,5-dibenzoate
A technology of dibenzoate and pentofuranose, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of unstable overall yield, high and low phase difference, no α-isomer purity, and harsh conditions, so as to facilitate the transformation , Reduce production costs, reduce the effect of risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] At room temperature, 150 mL of THF solvent was added to the reactor, and then 2-deoxy-2,2-difluoro-D-erythro-1-furanulose-3,5-dibenzoyl ester 20 g ( 0.054mol), sodium borohydride 2.2g (0.06mol) and PPO-CBS immobilized catalyst 2.0g, then, under agitation, slowly add methanol 6g dropwise, and control the temperature at 20°C to 30°C during the dropwise addition; After the addition is complete, continue to control the temperature at about 22°C for heat preservation reaction for 3 hours. After the reaction, add hydrochloric acid dropwise to the reaction solution to adjust the pH value of the system to 6.5, stir for 10 minutes, filter, and recover the solid PPO-CBS immobilized catalyst. Apply mechanically, the filtrate is left to stand for 30min, and then the organic phase is collected in layers; the aqueous layer can be extracted twice with 50mL of tetrahydrofuran, and the combined organic phase is washed once with 50mL of saturated aqueous sodium bicarbonate solution, left ...
Embodiment 2
[0024]At room temperature, 112 mL of THF solvent was added to the reactor, and then 20 g of 2-deoxy-2,2-difluoro-D-erythro-1-furanulose-3,5-dibenzoyl ester was added ( 0.054mol), sodium borohydride 2.2g (0.06mol) and PPO-CBS immobilized catalyst 1.8g, then, under stirring conditions, slowly add methanol 6g dropwise, and control the temperature at 20°C to 25°C during the dropwise addition; After the addition is complete, continue to control the temperature at about 22°C for heat preservation reaction for 3.5 hours. After the reaction, add hydrochloric acid dropwise to the reaction solution to adjust the pH value of the system to 6.5, stir for 10 minutes, filter, and recover the solid PPO-CBS immobilized catalyst. Apply mechanically, the filtrate is left to stand for 30min, and then the organic phase is collected in layers; the aqueous layer can be extracted twice with 50mL of tetrahydrofuran, and the combined organic phase is washed once with 50mL of saturated aqueous sodium bic...
Embodiment 3
[0026] At room temperature, 250 mL of THF solvent was added to the reactor, and then 20 g of 2-deoxy-2,2-difluoro-D-erythro-1-furanulose-3,5-dibenzoyl ester was added ( 0.054mol), sodium borohydride 2.4g (0.065mol) and PPO-CBS immobilized catalyst 2.2g, then, under the condition of stirring, slowly add methanol 8g dropwise, and control the temperature at 25°C to 30°C during the dropwise addition; After the addition is complete, continue to control the temperature at about 27°C for heat preservation reaction for 2.5 hours. After the reaction, add hydrochloric acid dropwise to the reaction solution to adjust the pH value of the system to 6.5, stir for 10 minutes, filter, and recover the solid PPO-CBS immobilized catalyst. Apply mechanically, the filtrate is left to stand for 30min, and then the organic phase is collected in layers; the aqueous layer can be extracted twice with 50mL of tetrahydrofuran, and the combined organic phase is washed once with 50mL of saturated aqueous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com