A kind of application of lanostane type
A lanostane-type, angiogenesis technology, which is applied to medical preparations containing active ingredients, pharmaceutical formulations, antitumor drugs, etc., can solve the problem of angiogenesis that has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 compound extraction, separation
[0024] 1. Extraction and separation
[0025] Dry and pulverize 2.0kg stems and leaves of King Kong, and soak 3 times with 95% ethanol, each time for 7 days, the extracts are combined, concentrated under reduced pressure to obtain extract (200g), and extracted with ethyl acetate to obtain the ethyl acetate part ( 80g). Use MCI-gel (30%-100%) for separation, wherein the second part is separated with silica gel, the eluent is petroleum ether-acetone (20:1-1:1), select the third part, and use reverse phase liquid Separated by phase chromatography, eluted with acetonitrile / water (64:36, 3mL / min), and collected in 27 minutes to obtain the compound.
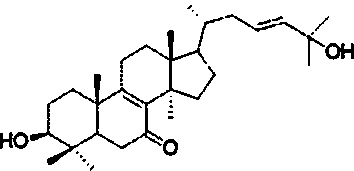
[0026] 2. Identification of the structure of the compound
[0027] white amorphous powder; 1 H NMR (400MHz, CDCl 3 ,δin ppm,J in Hz):δ H 3.29(1H,dd,J=4.6,11.6,H-3),5.59(1H,m,H-28a),5.59(1H,m,H-28b),0.75(3H,s,H-18), 1.08(3H,s,H-19),0.84(3H,d,J=6.5Hz,H-21),1.31(3H,s,H-26),1.31(3H,...
Embodiment 2
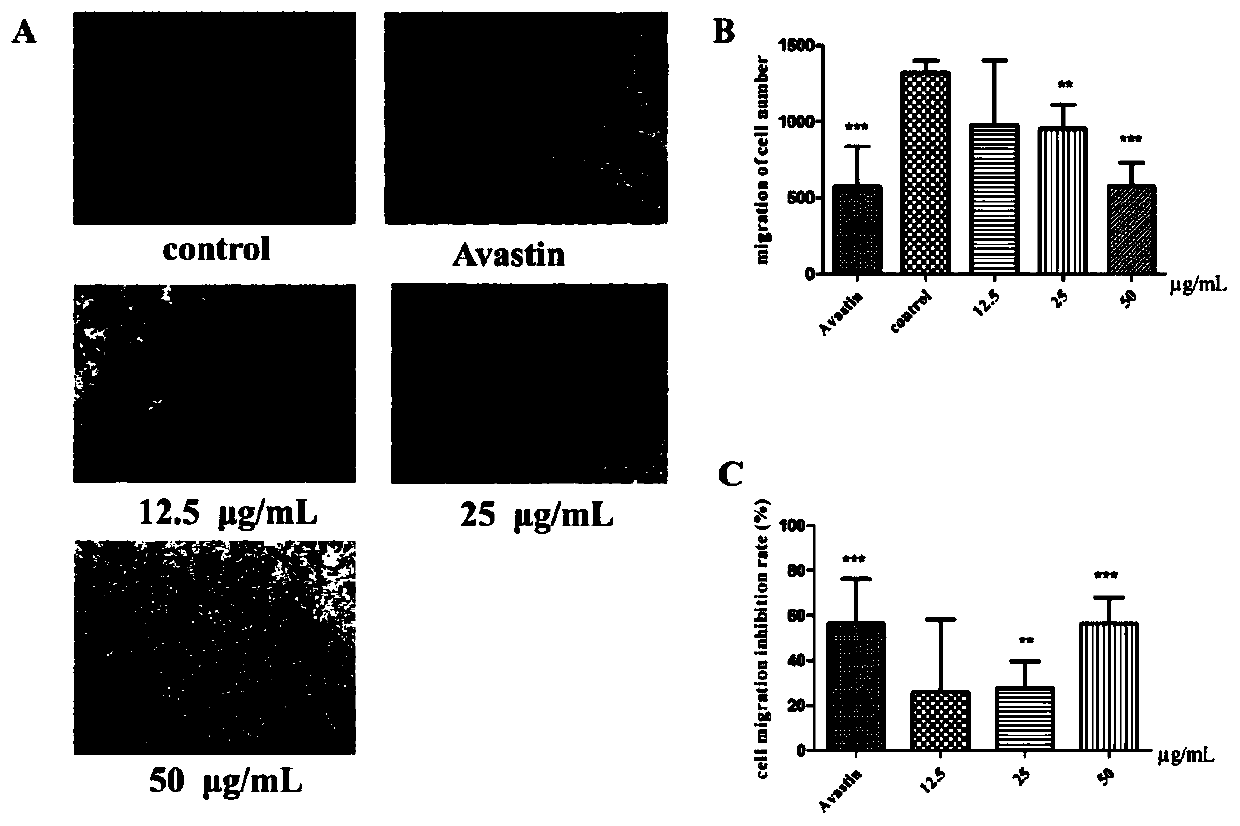
[0028] Inhibition of Example 2 Compounds on Migration of Endothelial Cells
[0029] 1. Experimental materials, instruments and reagents
[0030] 1 Experimental materials
[0031] 1.1 Drugs: Compounds (kansenonol) isolated in our laboratory
[0032] Avastin
[0033] 1.2 Cell lines:
[0034] Human umbilical vein endothelial cells (HUVEC) were preserved by our research group.
[0035] 2 Preparation methods of main test reagents
[0036] 2.1 DMEM preparation method: take 10.4g DMEM dry powder, 2g NaHCO 3 , 50mg penicillin, 100mg streptomycin, ddH 2 O 800mL, fully mixed with a magnetic stirrer, adjusted to pH 7.4 with HCl, sterilized by filtration with a 0.22μm filter, aliquoted, and stored at 4°C for later use.
[0037] 2.2 PBS liquid preparation method: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO4 1.44g, KH 2 PO 4 0.24g, add water to make up to 1L, sterilize under high temperature and high pressure, and store at 4°C for later use.
[0038] 2.3 Preparation method of trypsin: diss...
Embodiment 3
[0054] Inhibitory effect of embodiment 3 compound on rat arterial ring formation
[0055] 1. Experimental materials, instruments and reagents
[0056] 1 Experimental materials
[0057] 1.1 Drugs: Compounds (kansenonol) isolated in our laboratory
[0058] 1.2 Animals:
[0059] SD rats, SPF grade, female, body weight (250) g
[0060] 2 Preparation methods of main test reagents
[0061] 2.1 DMEM preparation method: take 10.4g DMEM dry powder, 2g NaHCO 3 , 50mg penicillin, 100mg streptomycin, ddH 2 O 800mL, fully mixed with a magnetic stirrer, adjusted to pH 7.4 with HCl, sterilized by filtration with a 0.22μm filter, aliquoted, and stored at 4°C for later use.
[0062] 2.2 PBS liquid preparation method: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.24g, add water to make up to 1L, sterilize under high temperature and high pressure, and store at 4°C for later use.
[0063] 2.3 Matrigel solution: Dilute Matrigel 1:1 with serum-free ECM medium.
[0064] 2. Exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com