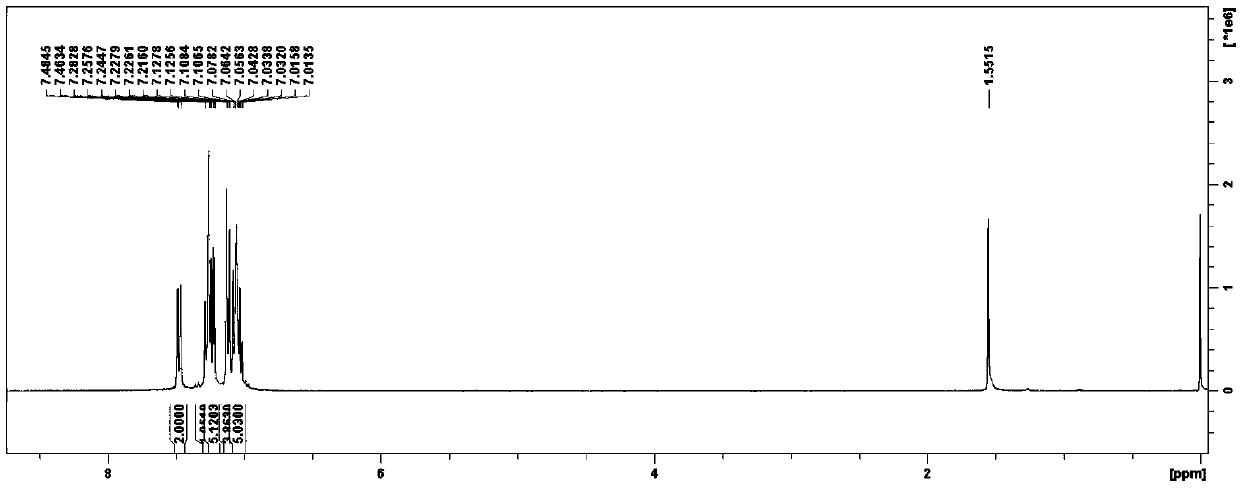
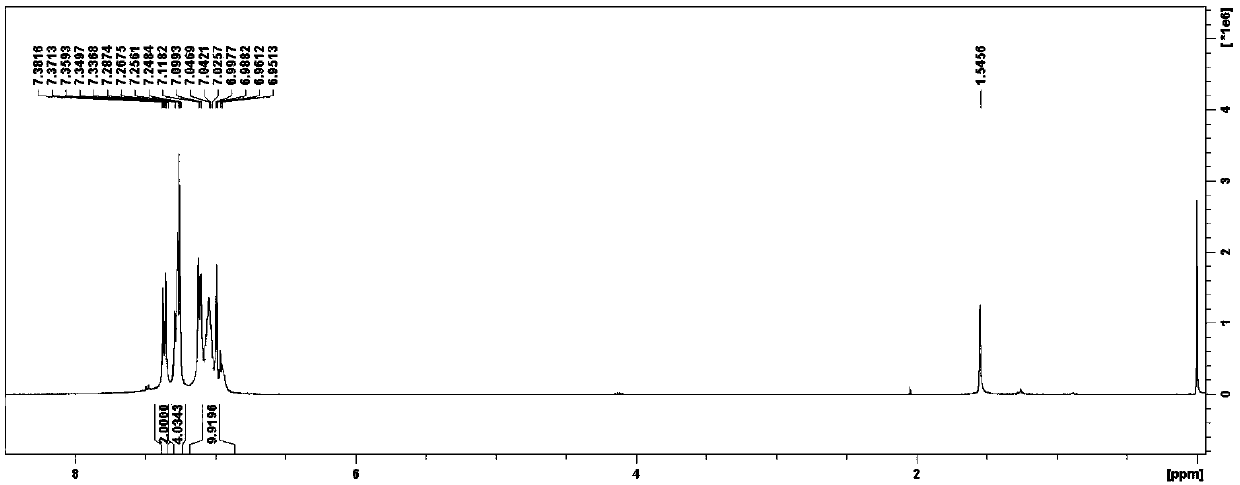
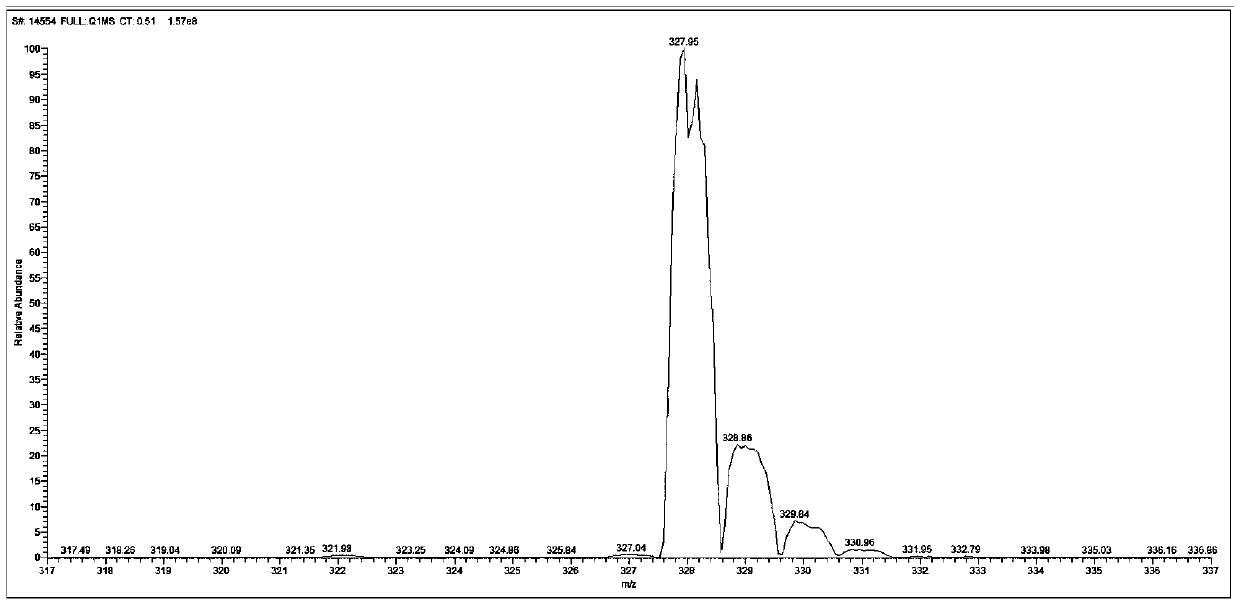
4-(2-thienyl)triphenylamine and derivatives thereof, and preparation method and application of 4-(2-thienyl)triphenylamine and derivatives thereof
A technology based on triphenylamine and thienyl, which is applied in the field of preparation of 4-triphenylamine and its derivatives, can solve the problems of poor UV absorption performance of ultraviolet absorbers, and achieve enhanced conjugation effect, high activity, and good chemical stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] A kind of 4-(2-thienyl) triphenylamine and derivative thereof, its preparation method specifically comprises the steps:
[0070] S1. First cover a layer of tin foil on the single-necked flask of the reaction vessel, and perform light-shielding treatment, then place 0.0025mol triphenylamine C1 in the single-necked flask that has been treated with light-shielding, add 40mL of chloroform, dissolve the reactant, and turn on the stirrer. And keep the state of 0 ℃ cold bath; After 15 minutes, add N-bromosuccinimide (generally speaking, NBS can be 0.00258~0.00275mol, in embodiment 1, be 0.0026 mol), after adding, keep stirring and cooling bath (generally, the cooling bath time can be 8~9h, in embodiment 1 is 8h);
[0071] After 8 hours, use a rotary evaporator to remove chloroform in the crude product, use a solution with a volume ratio of n-hexane and dichloromethane of 6:1 as an eluent, separate and purify through a silica gel chromatography column, collect the product, and ...
Embodiment 2
[0079] A kind of 4-(2-thienyl) triphenylamine and derivatives thereof, its preparation method is compared with the preparation method of embodiment 1, difference is that the halogenation reagent in embodiment 2 is N-iodosuccinimide , the reaction condition of the halogenation reaction in step S1. is -3°C cold bath for 8h under stirring;
[0080] Other raw material consumption and operating steps are consistent with embodiment 1.
Embodiment 3
[0082] A kind of 4-(2-thienyl) triphenylamine and its derivatives, its preparation method is compared with the preparation method of Example 1, the difference is that the temperature of Suzuki coupling reaction in the step S2. of Example 3 is 70 ℃ , the time is 48h;
[0083] Other raw material consumption and operating steps are consistent with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com