Exosome derived from human pluripotent stem cells, preparation based on exosome and use of exosome and preparation
A technology of human pluripotent stem cells and exosomes, applied in non-embryonic pluripotent stem cells, artificially induced pluripotent cells, embryonic cells, etc., can solve the problems of bone degenerative diseases and incapacitating diseases, and achieve large-scale applications Value, promoting tissue cell regeneration, and the effect of treating bone diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
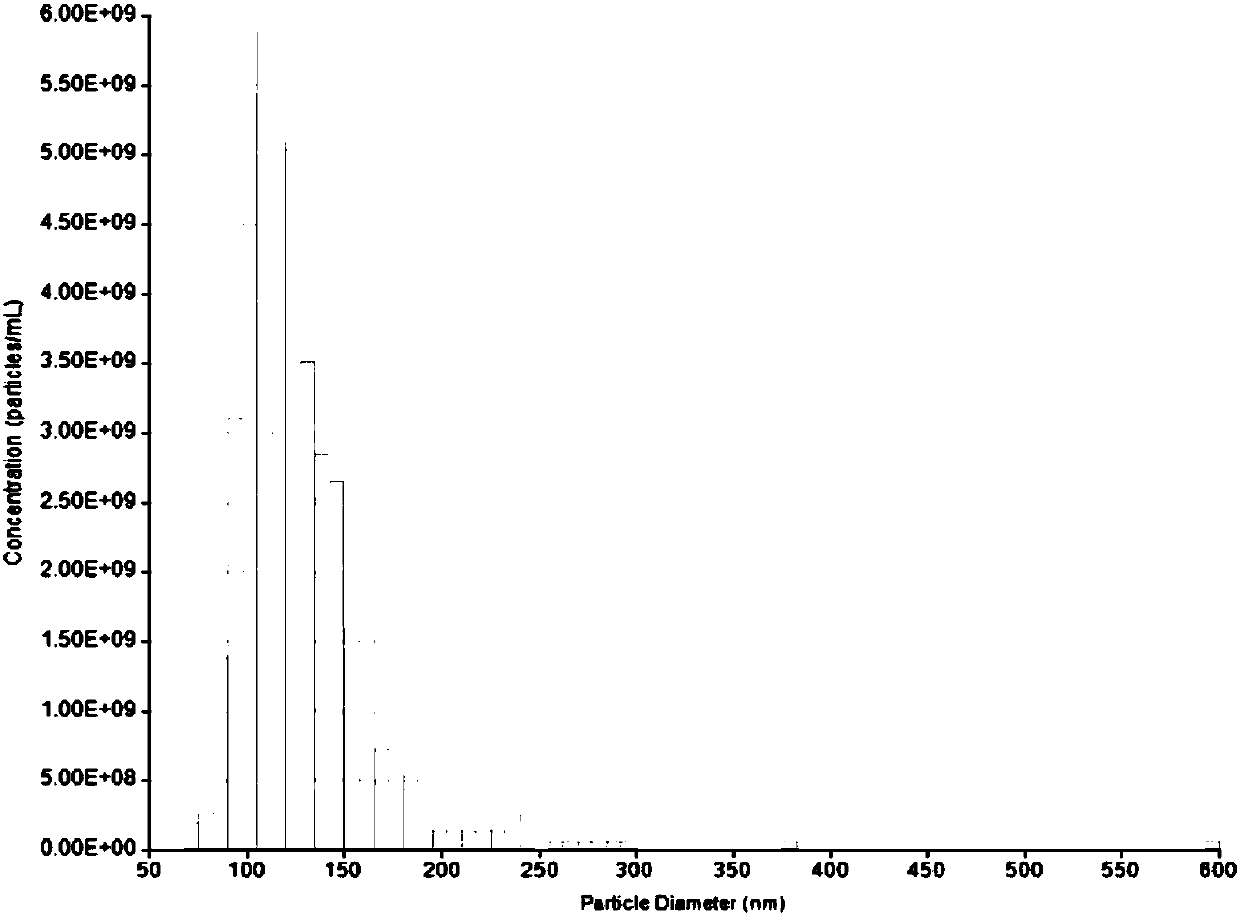
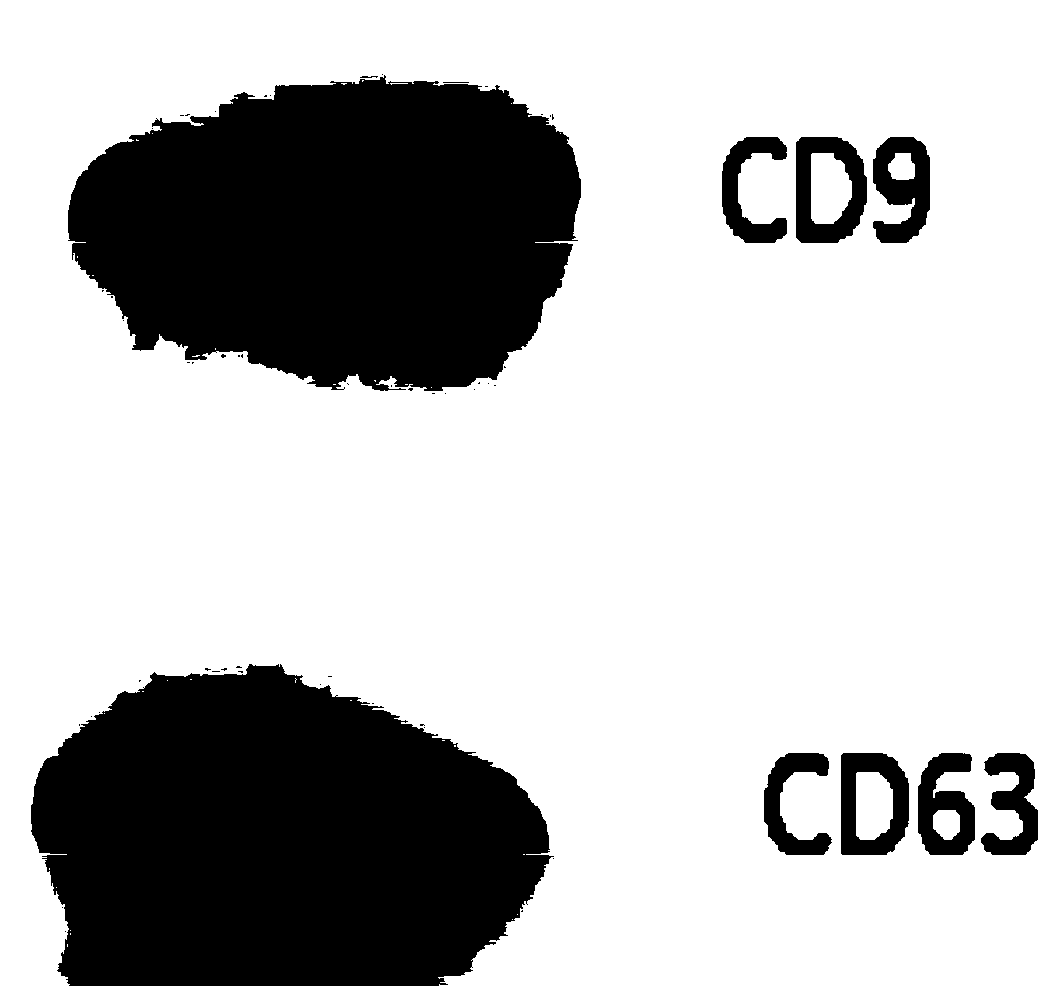
[0089] Culture of human embryonic stem cells (ESCs) and extraction and identification of exosomes
[0090] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), ESCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), in an incubator (37°C, 5% CO 2 , saturated humidity) culture, and collect the culture medium changed every day. Filter the medium through a 0.22 micron pore size filter membrane and centrifuge at 10,000g at 4°C for 30 minutes to remove cell debris; use a 100KD molecular weight ultrafiltration tube and centrifuge (3500g, 15min) to intercept exosomes in the concentrated supernatant to obtain exosomes concentrate; transfer the concentrate to a 30% sucrose / heavy water density pad (1.210 g / cm 3 ), centrifuge at 100,000g at 4°C for 210 minutes, collect the 5ml sucrose / heavy water density pad at the bottom, add PBS to dilute, transfer to an ultrafiltration centrifuge tube with a molecular weigh...
Embodiment 2
[0100] Prevention and treatment effect of exosomes derived from human pluripotent stem cells on osteoporosis
[0101] Prevention experiment: select the 6-month-old SAMP6 rapid aging mouse model, and divide the experiment into two groups, with 10 mice in each group. The experimental group is administered 2-5×10 8 Individual ES-exo or iPS-exo were intervened, and the control group was given the same volume of normal saline. The mice were killed 3 months after the intervention, and the samples were taken for testing.
[0102] Therapeutic experiment: select the 8-month-old SAMP6 rapid aging mouse model, divide the experiment into two groups, 10 mice in each group, and give human 2~9×10 8 Each ES-exo was intervened, and the control group was given the same volume of normal saline. The mice were killed 3 months after the intervention, and the samples were taken for testing.
[0103] Blood was collected from the mice before sacrifice, and the levels of serum tartrate-resistant aci...
Embodiment 3
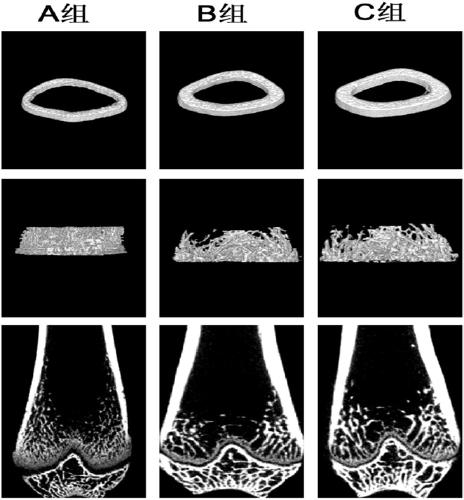
[0108] Preventive effect of exosomes derived from human embryonic stem cells on avascular necrosis of the femoral head
[0109] The culture of human embryonic stem cells and the extraction of exosomes are the same as in Example 1. In the experiment, 300-320 g male SD rats were used to make a model of hormone-induced necrosis of the femoral head: SD rats were intramuscularly injected with methylprednisolone (40 mg / kg), 3 times a week for 3 weeks. Experimental grouping: model group (hormone-induced necrosis of the femoral head, n=10); ES-exo intervention group (hormone-induced and simultaneous intravenous administration of ES-exo for intervention, n=10); normal control group (given the same dose of PBS, n=10). After all injections were completed, the rats were housed in cages and raised normally for 3 weeks. Then samples were taken to evaluate the neovascularization and osteonecrosis of the femoral head.
[0110] Detect changes in the bone structure of the femoral head by mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com