Collecting agent with amide group and thioamide group as well as preparation method and application thereof
A technology of thioamide and amide groups, applied in the field of collectors with amide groups and thioamide groups and their preparation, can solve the problems of no collector reports, achieve good selectivity, and enhance chelation performance , Improve the effect of harvesting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
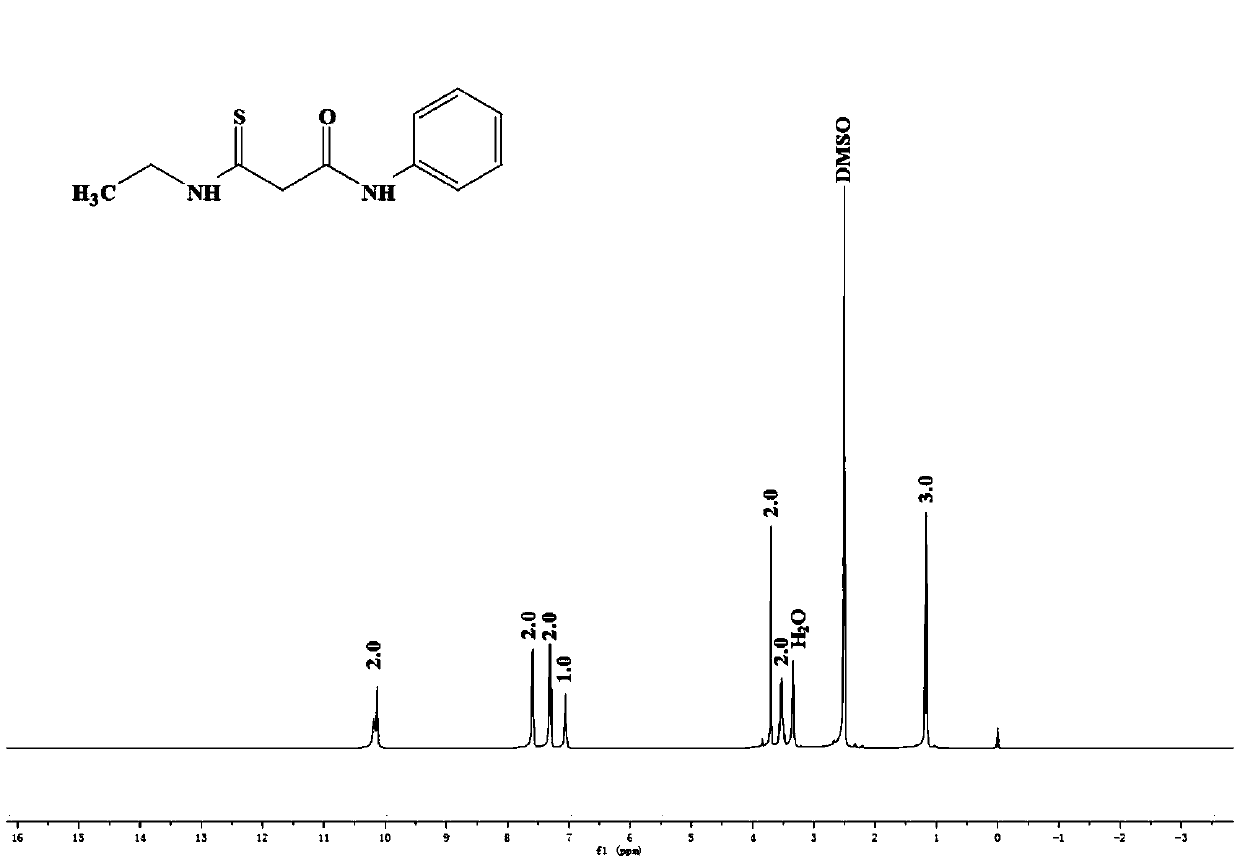
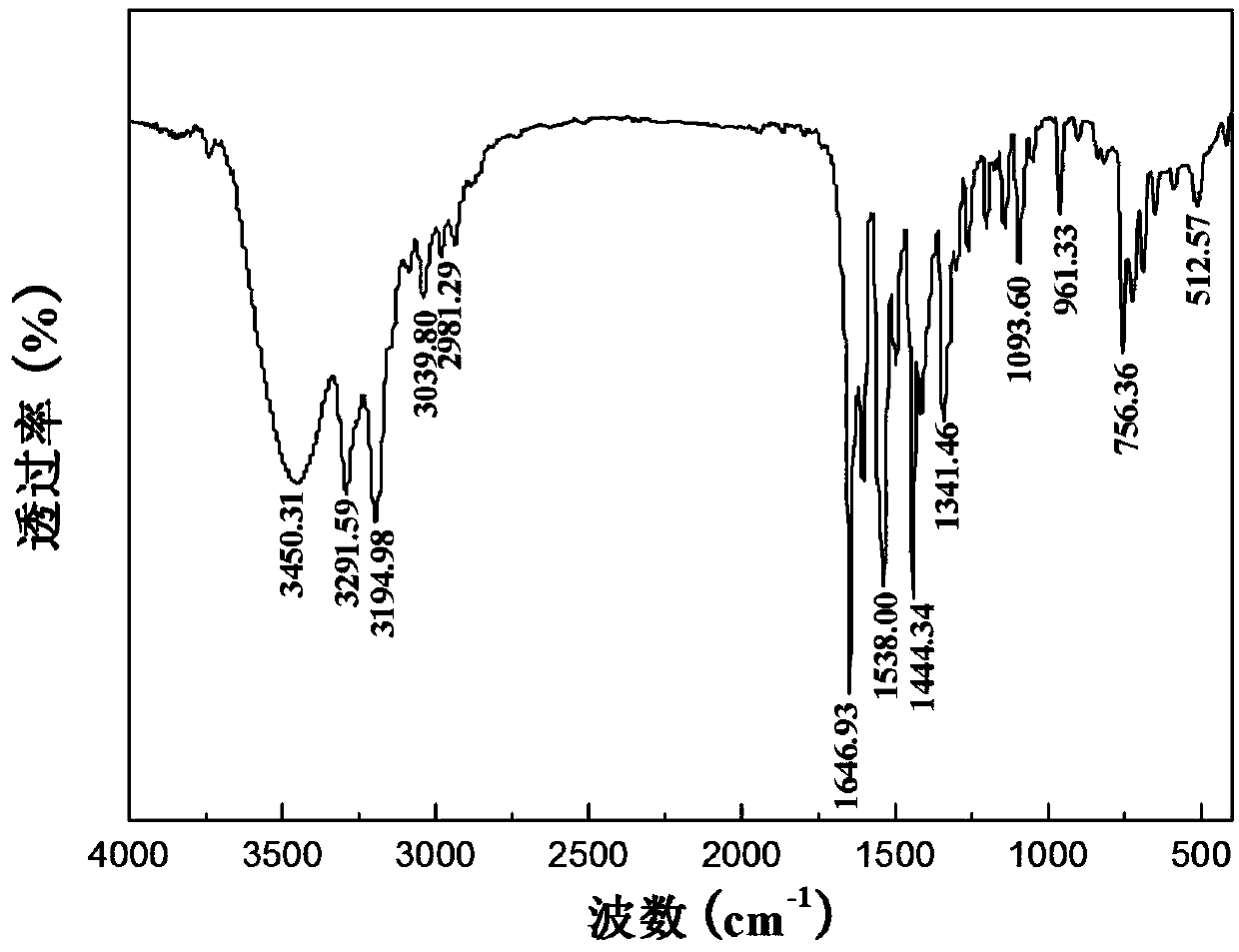
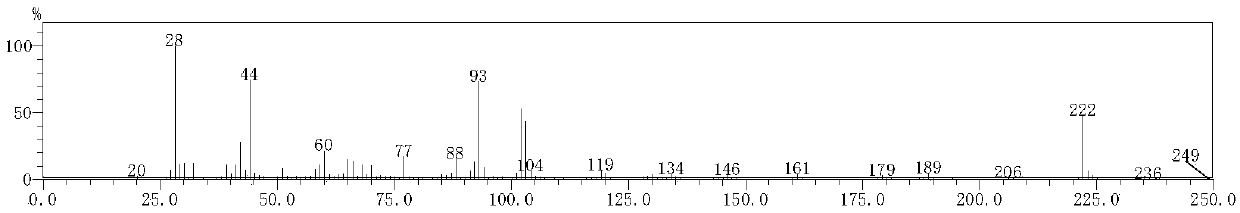
[0037] The preparation of 3-ethylamino-N-phenyl-3-oxygenide thiopropionamide: 1.04g purity is 95% ethyl isothiocyanate, 1.38g purity is 99% potassium carbonate, 1.77g Add acetoacetanilide with a purity of 98% to 30 mL of absolute ethanol with a purity of 99.7%, stir at room temperature for 30 min, heat the system to reflux, and stir at room temperature for 2.5 h. After the reaction, distill off the ethanol, add 30mL of 10% dilute hydrochloric acid after cooling, stir, filter the resulting precipitate, wash with water, and dry in vacuum to obtain 3-ethylamino-N-phenyl-3-oxylidenethiopropane Amide, the yield (based on acetoacetanilide) is 91.2%, and the proton nuclear magnetic resonance spectrum is as figure 1 As shown, the infrared spectrum as figure 2 As shown, the mass spectrum is as image 3 shown, from Figure 1-3The synthesis of 3-ethylamino-N-phenyl-3-oxylidenethiopropionamide can be further illustrated.
Embodiment 2
[0039] The preparation of 3-ethylamino-N-(tolyl)-3-oxygenide thiopropionamide: 1.91g purity is 99% 2-methyl acetoacetanilide, 1.38g purity is 99% potassium carbonate 1.62 g of phenyl isothiocyanate with a purity of 98% was added to 30 ml of absolute ethanol with a purity of 99.7%, stirred at room temperature for 30 min, the system was heated to reflux, and stirred at room temperature for 2 h. After the reaction, distill ethanol off, add 30mL of 10% dilute hydrochloric acid after cooling, stir, filter the resulting precipitate, wash with water, and dry in vacuo to obtain 3-ethylamino-3-(methylphenyl)-3-oxylidene Thiopropionamide, the yield (based on 2-methylacetoacetanilide) was 84.2%. H NMR spectrum such as Figure 4 shown. from Figure 4 The synthesis of 3-ethylamino-N-(tolyl)-3-oxylidenethiopropionamide could be further confirmed.
Embodiment 3
[0041] The preparation of 3-anilino-N, N-dimethyl-3-oxygenide thiopropionamide: 1.62g purity is 98% phenyl isothiocyanate, 1.38g purity is 99% potassium carbonate, 1.29 g of N,N-dimethylacetoacetamide with a purity of 98% was added to 30 mL of absolute ethanol with a purity of 99.7%, stirred at room temperature for 30 min, the system was heated to reflux, and stirred at room temperature for 1.8 h. After the reaction, distill off the ethanol, add 30mL of 10% dilute hydrochloric acid after cooling, stir, filter the resulting precipitate, wash with water, and dry in vacuum to obtain 3-anilino-N,N-dimethyl-3-oxyethylenesulfur On behalf of propionamide, the yield (based on N,N-dimethylacetoacetamide) is 88.6%, and the proton nuclear magnetic resonance spectrum is as follows: Figure 5 shown. from Figure 5 The synthesis of 3-anilino-N,N-dimethyl-3-oxylidenethiopropionamide can be further illustrated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com