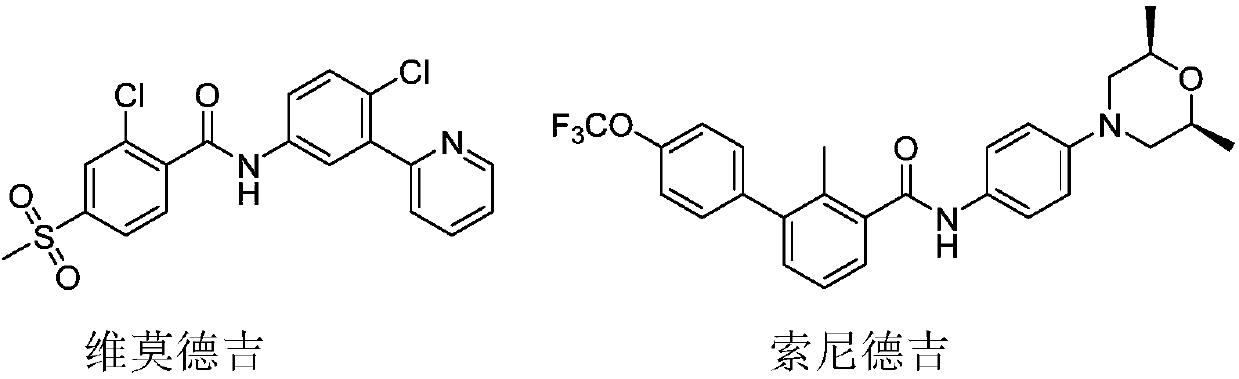
Compound as well as preparation method and application thereof
A compound and composition technology, applied in the fields of compound and its preparation and application, can solve the problems of toxic and side effects, drug resistance, not being approved for medulloblastoma, etc., and achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046]
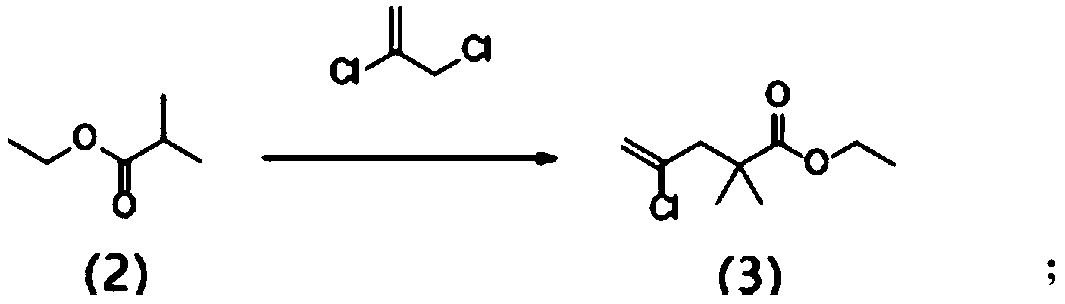
[0047] (1) Lithium diisopropylamide (LDA, 150mL, 2mol, in tetrahydrofuran) was added dropwise to tetrahydrofuran (500mL), cooled to -78°C, ethyl isobutyrate (formula (2), 33g , 0.284mol), the reaction mixture was kept at -70 to -65°C for 20 minutes, and then 2,3-dichloropropene (31 g, 0.279mol) was added dropwise. After the addition was completed, the reaction solution was gradually warmed to room temperature for 18 hours After the reaction was completed, it was quenched with saturated ammonium chloride, extracted twice with dichloromethane (2×300mL), the combined organic layer was dried over anhydrous sodium sulfate, filtered and rotary evaporated to obtain a brown oil compound 4-chloro-2, Ethyl 2-dimethyl-4-pentenoate (formula (3), 59 g, 0.279 mol, 93% yield) was directly used in the next reaction. Formula (3) 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 1.24-1.28 (m, 9H), 2.65 (s, 2H), 4.15 (q, 4H), 5.13 (s, 1H), 5.24 (s, 1H).
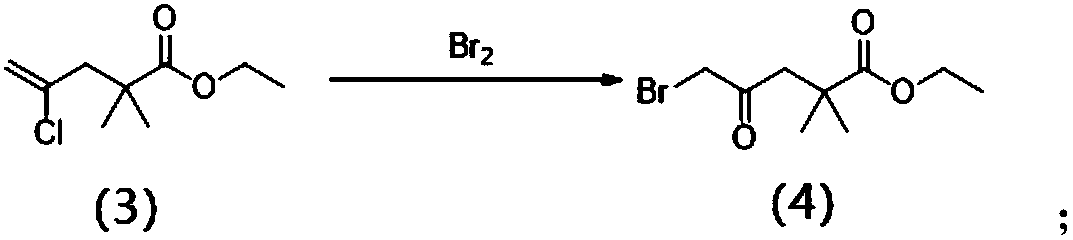
[0048] (2) 4-chloro-2,2-dime...
Embodiment 2
[0056]
[0057] According to the method described in Embodiment 1, the difference between the method described in Embodiment 2 and the method described in Embodiment 1 is that the following steps (9) and (10) are used to replace the steps in Embodiment 1 ( 8):
[0058] (9) 3-(3-(tert-butylmercapto)-5-isopropyl-1-(4-methoxybenzyl)-1 hydrogen-indol-2-yl) obtained in step (7) -Ethyl 2,2-dimethylpropionate (formula (11), 1.5 g, 3 mmol) and tert-butylmercaptan (t-BuSH, 2.7 g, 30 mmol) were dissolved in CH 2 Cl 2 (40 mL), at 0°C, add AlCl in portions within 5 minutes 3 (2.0g, 15mmol), the reaction mixture was reacted at this temperature for 2h, poured into ice water after the reaction was completed, added 1 equivalent of HCl, extracted twice with dichloromethane, and the organic phase was washed with water, brine and anhydrous sulfuric acid successively. Na-dried, concentrated and purified by silica gel column (EA (ethyl acetate): PE (petroleum ether) = 10:1) to obtain the whi...
Embodiment 3
[0062]This example is used to illustrate the effect of the compound of formula (1) of the present invention on tumor proliferation associated with the activation of hedgehog pathway.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com