Novel indole hemicyanine-based SO3<2-> ratiometric fluorescent probe and applications thereof
A ratiometric fluorescent probe, SO32- technology, applied in fluorescence/phosphorescence, luminescent materials, material analysis by optical means, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: The synthesis scheme of the compound of formula (1) is shown in the following formula:
[0019]
[0020] Concrete synthetic steps are as follows:
[0021] In a 25 mL round bottom flask, add successively) 0.29 g (1.0 mmol) of 4-phenyl-pyrido[1,2-a]benzimidazole-3-carboxylic acid, 0.56 g (1.2 mmol) of 3,3-di Methyl-1-ethyl-2-(4-(piperazinyl)-styryl) indole iodide, 0.18g (1.5mmol) DMAP, 0.29g (1.5mmol) EDC, 20ml dichloromethane, room temperature React for 24 hours. After the reaction was detected by TLC, the heating was stopped, and about 1 g of silica gel was added after cooling to room temperature, concentrated and dried on a vacuum rotator, sampled by dry method, separated by column chromatography, and concentrated to obtain 0.64 g of a purple solid with a yield of 86.5%.
[0022] H NMR spectrum determination: 1 H NMR (400 MHz, CDCl 3 ): δ 8.57 (d, 1H), 8.20 (d, 2H), 8.0 (m, 3H), 7.87 (d, 2H), 7.65 (d, 1H), 7.50 (ddd, 9H), 6.95 (d, 1H ), 6.82 (d,2H...
Embodiment 2
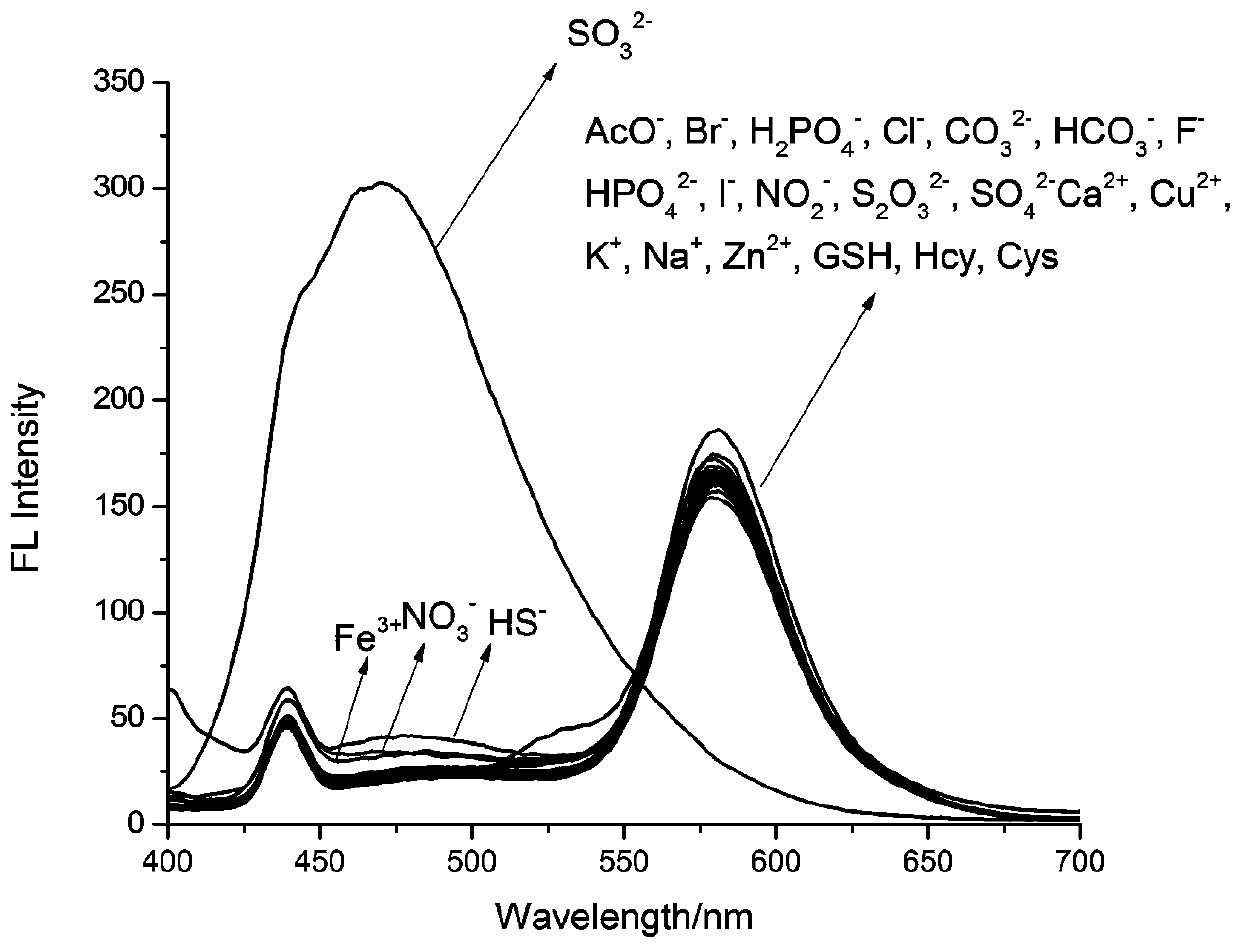
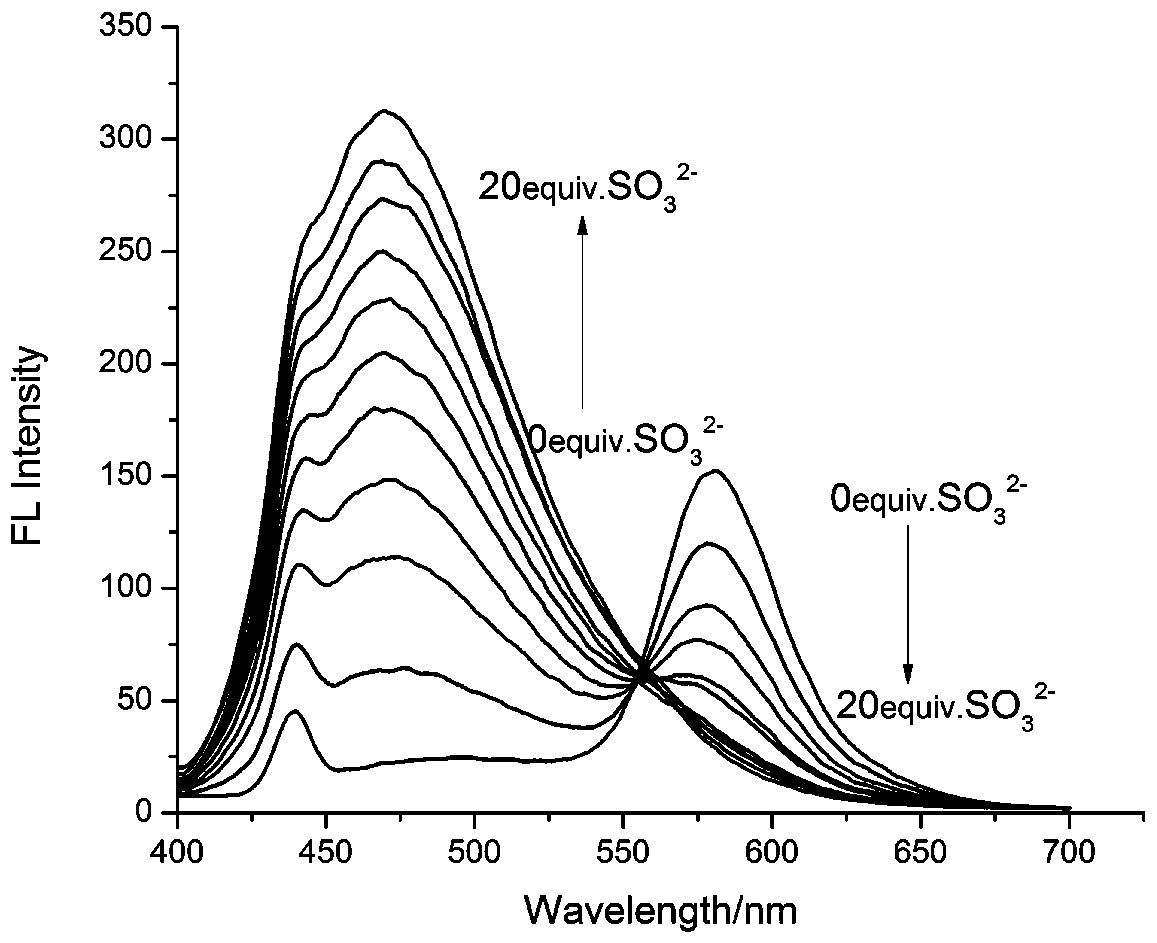
[0024] To the compound of formula (1) (5×10 -5 M) were added to the neutral solution of 10 equivalents of SO 3 2- , AcO - , Br - , H 2 PO 4 - , Cl - , CO 3 - , HCO 3 - , F - , HPO 4 2- , HS - , I - , NO 2 - , NO 3 - , S 2 o 3 2- , SO 4 2- , Fe 3+ , Ca 2+ , Cu 2+ , K + , Na + , Zn 2+, GSH, Hcy and Cys, measured the changes in the ratio of fluorescence emission intensity at 470nm and 578nm and found that: the compound of formula (1) has 3 2- Has a unique fluorescence selectivity, adding 10 equivalents of SO 3 2- Afterwards, the fluorescence intensity of compound 1 at 578nm was significantly reduced, and at the same time, the fluorescence intensity at 470nm was significantly enhanced, I 470 / I 578 = 7.19, such as figure 2 shown.
Embodiment 3
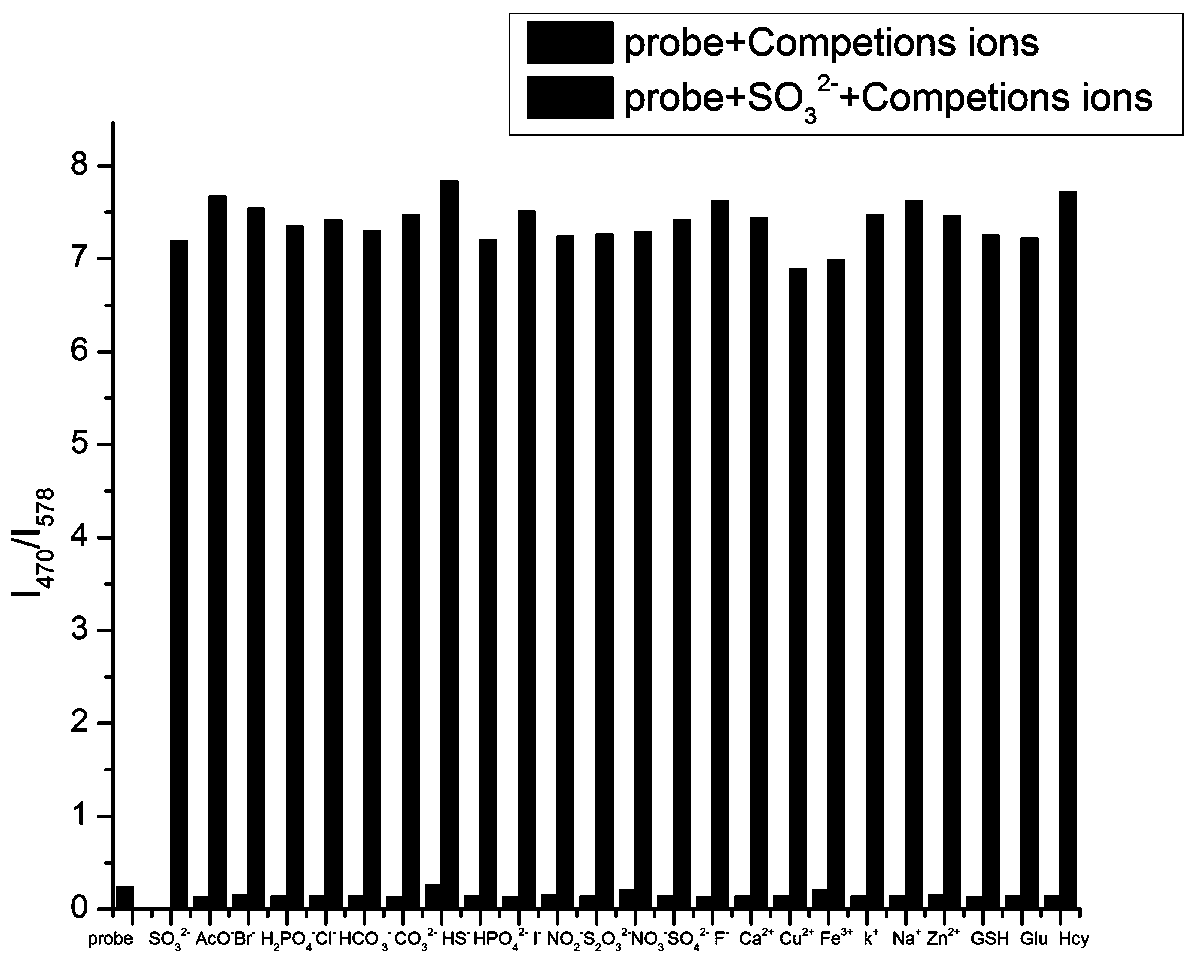
[0026] Formula (1) compound (5 × 10 -5 M) Add 10 equivalents of SO to the neutral aqueous solution 3 2- , AcO - , Br - , H 2 PO 4 - , Cl - , CO 3 - , HCO 3 - , F - , HPO 4 2- , HS - , I - , NO 2 - , NO 3 - , S 2 o 3 2- , SO 4 2- , Fe 3+ , Ca 2+ , Cu 2+ , K + , Na + , Zn 2+ , GSH, Hcy and Cys, measured the changes in the fluorescence emission curves at 470nm and 578nm and found that the compound of formula (1) has strong anti-interference ability to other ions, such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com