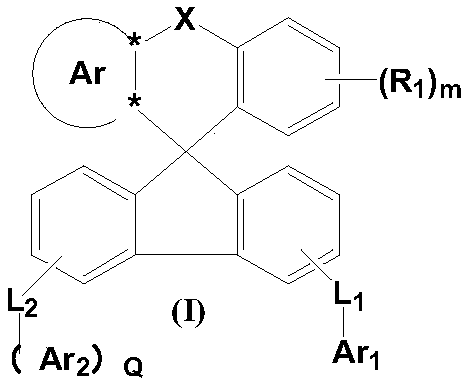
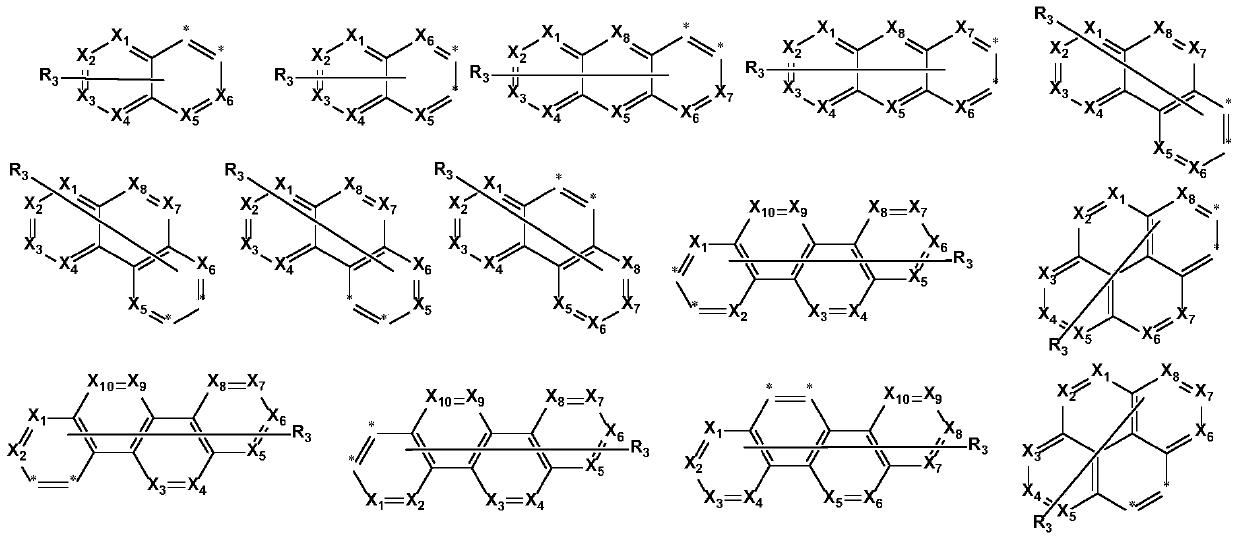
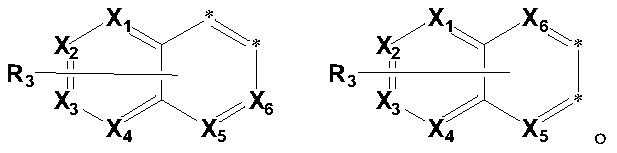
Fused ring organic compound and mixture and organic electronic device comprising same
A technology of organic compounds and mixtures, which is applied in the field of organic electronic devices and fused-ring organic compounds, can solve the problems of efficiency, lifespan and thermal stability, etc., and achieve excellent electron transport properties and stability, simple material synthesis, and improved electrical efficiency. The effect of luminescence efficiency and device lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0256] According to the above-mentioned preparation method, a functional layer is formed with a thickness of 5nm-1000nm.
[0257] The present invention also relates to the use of the above-mentioned condensed ring organic compound or mixture in organic electronic devices.
[0258] The present invention further relates to an organic electronic device comprising a condensed ring organic compound or high polymer or mixture as described above.
[0259] The organic electronic device can be selected from, but not limited to, organic light emitting diode (OLED), organic photovoltaic cell (OPV), organic light emitting cell (OLEEC), organic field effect transistor (OFET), organic light emitting field effect transistor, organic Lasers, organic spintronic devices, organic sensors and organic plasmon emitting diodes (Organic Plasmon Emitting Diode), etc., are particularly preferred organic electroluminescent devices, such as OLED, OLEEC, organic light emitting field effect tube.
[0260]...
Embodiment 1
[0277]
[0278] Synthesis of intermediate 3: 30 g of 1-naphthol (intermediate 1), 59 g of o-bromoiodobenzene (intermediate 2) were dissolved in 300 mL of dry THF, and 8 g of NaH was added. Under nitrogen atmosphere, stir at room temperature for 12h. Carefully add methanol to quench the reaction, wash with water to separate the liquid, and obtain intermediate 3. MS (ASAP): 299.17 by column chromatography.
[0279] Synthesis of Intermediate 4: Dissolve 40g of Intermediate 3 in 400mL of dry THF, slowly drop into 66mL of 2M n-BuLi at -78°C under nitrogen atmosphere, stir for 1 hour, and slowly drop into Intermediate 4 (35g) THF solution. Then return to room temperature. The reaction was quenched by adding water, washed with water to separate the liquid, and separated by column chromatography to obtain the crude product. After drying, dissolve in 300 mL of a mixed solvent of glacial acetic acid and hydrochloric acid (glacial acetic acid: hydrochloric acid = 10:1 (volume ratio...
Embodiment 2
[0283]
[0284] Synthesis of Comp 2: 50 g of Intermediate 6, 34 g of Intermediate 8 and 4.5 g of Pd (PPh 3 ) 4 Dissolved in 600mL toluene, refluxed under nitrogen atmosphere for 12h. The solvent was spin-dried, extracted and separated, and recrystallized to obtain compound Comp 2.MS (ASAP): 689.82.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com