Application of biscyclopentadienyl rare-earth metal complex in catalysis of dehydrogenated coupling of amine broane
A technology for catalyzing amine boranes and rare earth metals, applied in organic compound/hydride/coordination complex catalysts, metallocenes, physical/chemical process catalysts, etc., can solve the problem of difficult to control hydrogen release rate, low atom economy, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
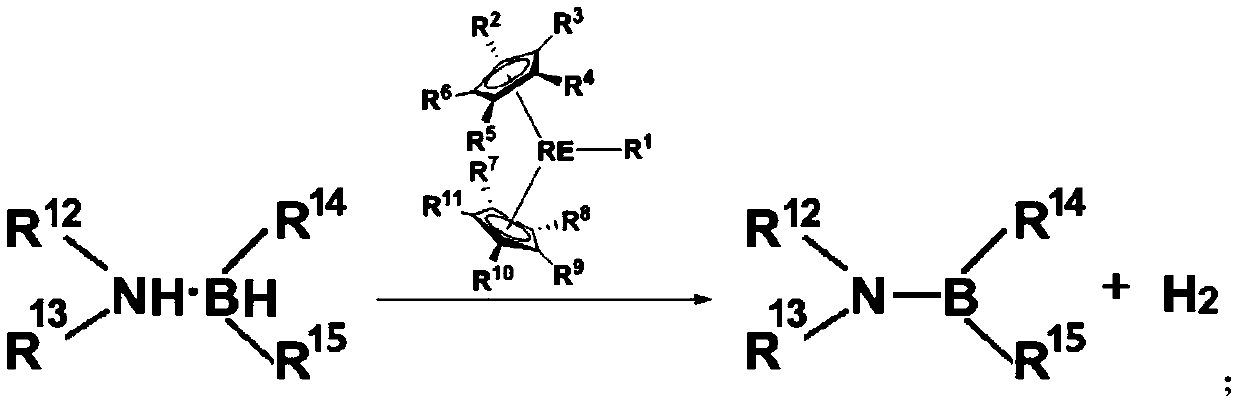
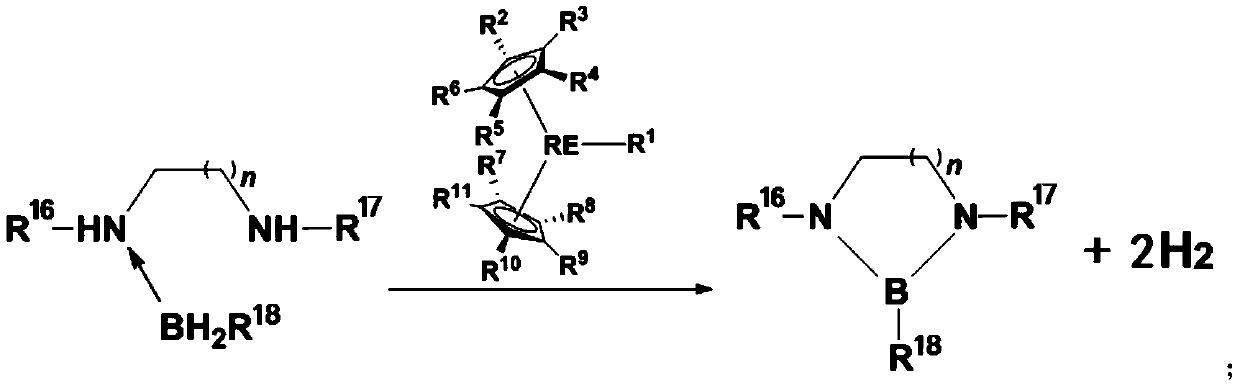
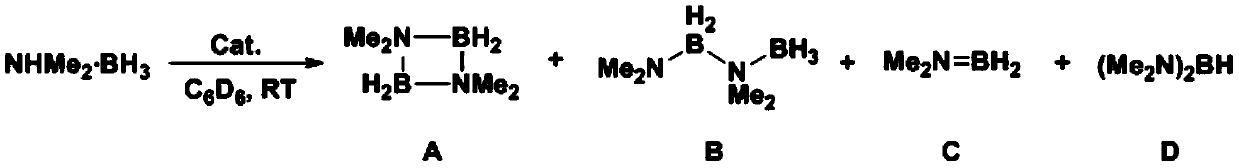
[0059] This example provides a method for preparing a dehydrogenated coupling product of amine borane, and its reaction route and specific steps are as follows (wherein, Me represents a methyl group, Cat represents a catalyst, and RT represents room temperature (about 20-30°C) , C 6 D. 6 represents deuterated benzene):
[0060]
[0061] Weigh Cp in the glove box * 2 ScCH(SiMe 3 ) 2 (Cp * =C 5 Me 5 ) 1.4 mg was dissolved in 100 μL deuterated benzene. Weigh 35.3mg dimethylaminoborane (NMe 2 H.BH 3 ), add 400 μL of toluene to dissolve, and quickly add it to the above solution. A large number of hydrogen bubbles were observed immediately, and no obvious bubbles were produced after 2 minutes, and about 0.2 μL was quickly taken out and added to an NMR tube, and 0.5 mL of undried deuterated benzene was added to it to quench the reaction. They were then frozen in liquid nitrogen and thawed until NMR analysis. Subsequently, the raw material conversion rate and product d...
Embodiment 2
[0068] This embodiment provides a method for preparing a dehydrogenated coupling product of amine borane, the reaction scheme is the same as in Example 1, and the specific steps are as follows:
[0069] Weigh Cp in the glove box * 2 ScCH(SiMe 3 ) 2 (Cp * =C 5 Me 5 ) 1.4 mg was dissolved in 100 μL deuterated benzene. Weigh 88.3mg dimethylaminoborane (NMe 2 H.BH 3 ), add 400 μL of toluene to dissolve, and quickly add it to the above solution. A large number of hydrogen bubbles were observed immediately, and no obvious bubbles were produced after 4 minutes, and about 0.2 μL was quickly taken out and added to an NMR tube, and 0.5 mL of undried deuterated benzene was added to it to quench the reaction. They were then frozen in liquid nitrogen and thawed until NMR analysis. Then, the raw material conversion rate and product distribution were analyzed by nuclear magnetic boron spectroscopy, and the raw material conversion rate was measured to be 97.4%, and the calculated TO...
Embodiment 3
[0073] This embodiment provides a method for preparing a dehydrogenated coupling product of amine borane, the reaction scheme is the same as in Example 1, and the specific steps are as follows:
[0074] Weigh Cp in the glove box * 2 LuCH (SiMe 3 ) 2 (Cp * =C 5 Me 5 ) 1.8 mg was dissolved in 100 μL deuterated benzene. Weigh 35.3mg dimethylaminoborane (NMe 2 H.BH 3 ), add 400 μL of toluene to dissolve, and quickly add it to the above solution. Immediately observe the generation of a large number of hydrogen bubbles, wait until 6 minutes when no obvious bubbles are generated, quickly take out about 0.2 μL from it and add it to the NMR tube, and add 0.5 mL of undried deuterated benzene to it to quench the reaction. They were then frozen in liquid nitrogen and thawed until NMR analysis. Subsequently, the raw material conversion rate and product distribution were analyzed by nuclear magnetic boron spectroscopy, and the measured raw material conversion rate was 100%, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com