Nickel-copper tungstate nano heterojunction particle, preparation method thereof and application of nickel-copper tungstate nano heterojunction particle in catalytic hydrogen production
A copper nano-heterojunction technology, applied in the direction of nickel compounds, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of catalyst particle agglomeration, reduce cycle life, etc., achieve regular shape and improve metal dispersion , the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of nickel-copper tungstate nano-heterojunction particles, comprising the following steps:
[0033] S1. Dissolve 1.6mmol of nickel nitrate and 0.4mmol of copper nitrate in 40mL of ultrapure water and stir for 5min to prepare mixed salt solution A;
[0034] S2. Dissolve 2mmol sodium tungstate in 40mL ultrapure water to form solution B;
[0035] S3. Add 4mmol sodium salicylate into the solution A, stir and dissolve, slowly add the solution B dropwise through a separatory funnel, and stir for 5min to obtain a solution C;
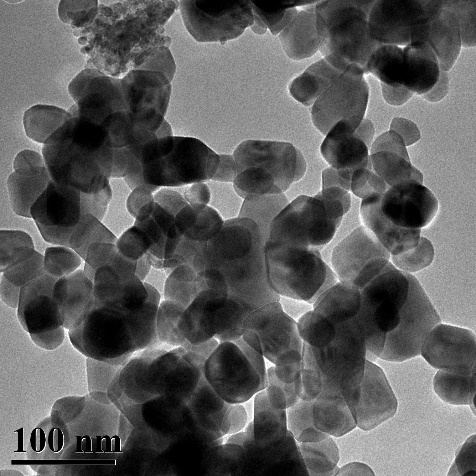
[0036] S4. Move the solution C to a 100mL reaction kettle, react at 170°C for 5h, filter and wash the solid at the bottom of the reaction kettle, then move it to a muffle furnace, and react at 500°C for 2h to obtain Ni 0.8 Cu 0.2 WO 4 Nickel-copper tungstate nano-heterojunction, its morphology is as follows figure 1 shown.
[0037] The prepared nickel-copper tungstate nano-heterojunction particles Ni 0.8 Cu 0.2 WO 4 Used as a ...
Embodiment 2
[0039] A preparation method of nickel-copper tungstate nano-heterojunction particles, comprising the following steps:
[0040] S1. Dissolve 1.2mmol of nickel nitrate and 0.8mmol of copper nitrate in 40mL of ultrapure water and stir for 10min to prepare mixed salt solution A;
[0041] S2. Dissolve 2mmol sodium tungstate in 40mL ultrapure water to form solution B;
[0042] S3. Add 4mmol sodium salicylate into the solution A, stir and dissolve, slowly add the solution B dropwise through a separatory funnel, and stir for 5min to obtain a solution C;
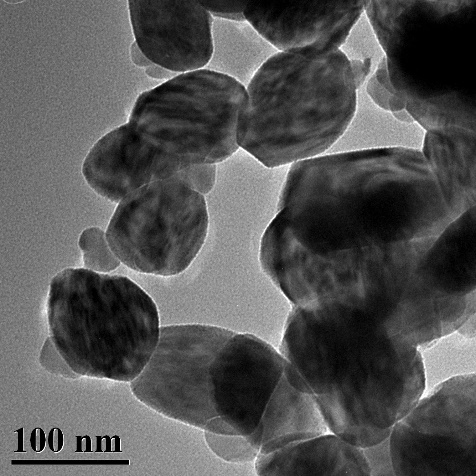
[0043] S4. Move the solution C to a 100mL reaction kettle, react at 170°C for 5h, filter and wash the solid at the bottom of the reaction kettle, then move it to a muffle furnace, and react at 500°C for 2h to obtain Ni 0.6 Cu 0.4 WO 4 Nickel-copper tungstate nano-heterojunction, its morphology is as follows figure 2 shown.
[0044] The prepared nickel-copper tungstate nano-heterojunction particles Ni0.6 Cu 0.4 WO 4 Used as a ...
Embodiment 3
[0046] A preparation method of nickel-copper tungstate nano-heterojunction particles, comprising the following steps:
[0047] S1. Dissolve 0.8mmol of nickel nitrate and 1.2mmol of copper nitrate in 40mL of ultrapure water and stir for 10min to prepare mixed salt solution A;
[0048] S2. Dissolve 2mmol sodium tungstate in 40mL ultrapure water to form solution B;
[0049] S3. Add 4mmol sodium salicylate into the solution A, stir and dissolve, slowly add the solution B dropwise through a separatory funnel, and stir for 5min to obtain a solution C;
[0050] S4. Move the solution C to a 100mL reaction kettle, react at 170°C for 5h, filter and wash the solid at the bottom of the reaction kettle, then move it to a muffle furnace, and react at 500°C for 2h to obtain Ni 0.4 Cu 0.6 WO 4 Nickel-copper tungstate nano-heterojunction, its morphology is as follows image 3 shown.
[0051] The prepared nickel-copper tungstate nano-heterojunction particles Ni 0.4 Cu 0.6 WO 4 Used as a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com