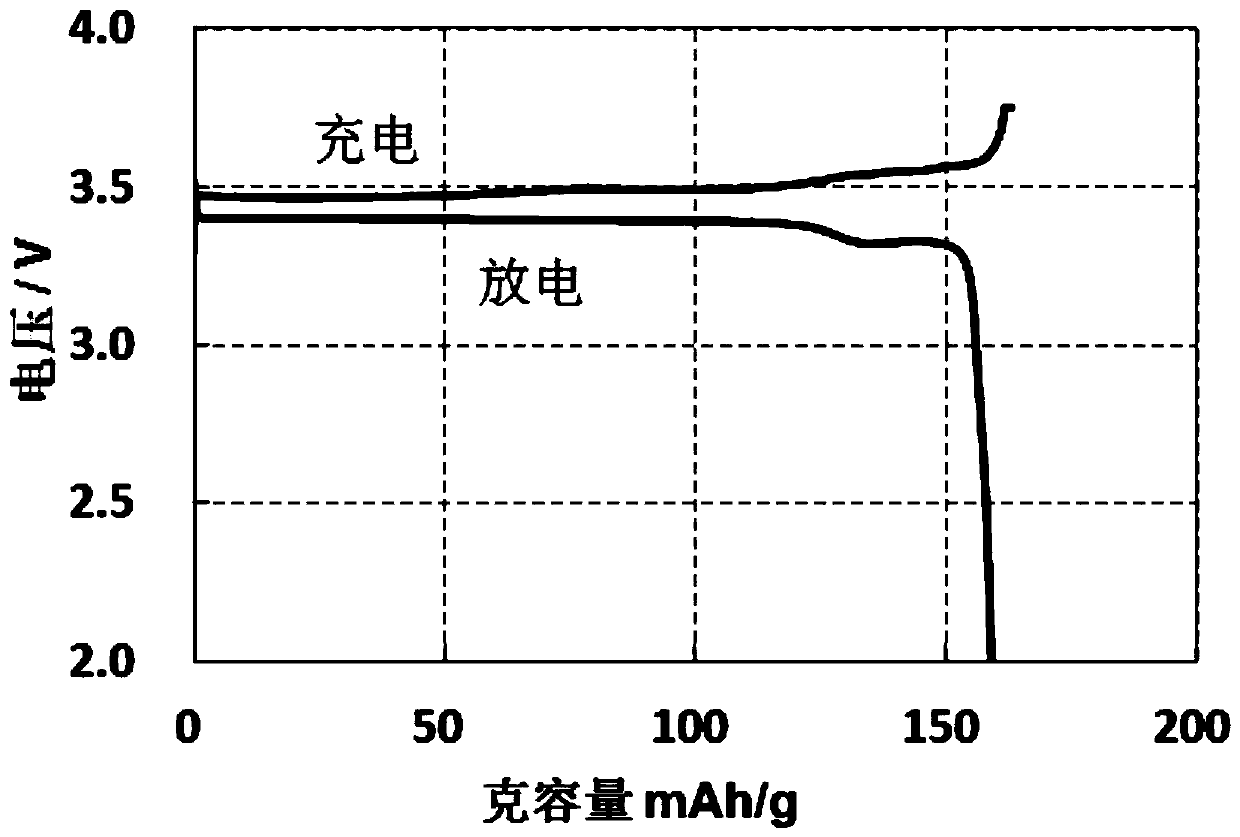
Method for recycling lithium iron phosphate positive electrode material
A technology of lithium iron phosphate and cathode material, applied in the field of electrochemistry, can solve the problems of resource waste, environmental pollution, etc., and achieve the effects of saving recovery costs, simple recovery methods, and good overall performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Step a) Disassemble a 35Ah lithium iron phosphate hard-shell battery whose capacity has decayed to 80%, cut off the tabs, and put the winding core (~600g) and residual electrolyte (~20g) into 1.5L of hydrochloric acid with a concentration of 5M solution, then add 250ml 3M hydrogen peroxide solution, and stir and soak for 2h at 25°C.
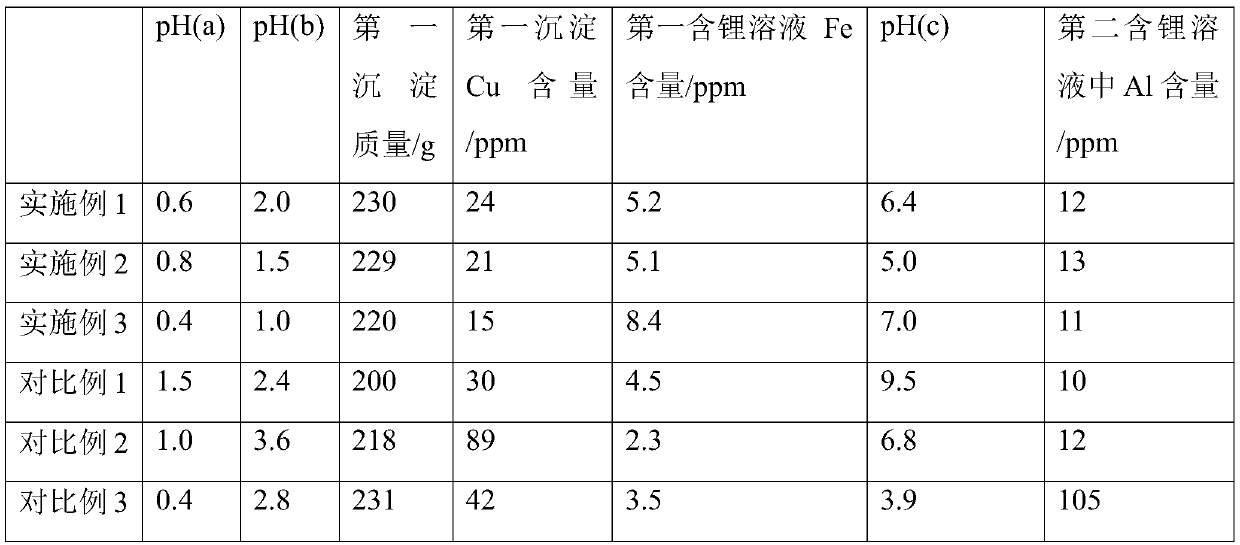
[0050] Sieve the above mixture through a 100-mesh screen to remove insoluble impurities to obtain a suspension; filter the suspension under reduced pressure to obtain a recovered carbon source and the first liquid phase, the pH of the first liquid phase pH(a) = 0.6.
[0051] Step b) Add 5M ammonia solution dropwise to the above solution until pH(b)=2.0, and filter to generate the first precipitate (~230g) whose main component is iron phosphate and the first lithium-containing solution.
[0052] Step c) Continue to add 5M aqueous ammonia solution dropwise to the first lithium-containing solution until pH (c)=6.4, and obtain the second lith...
Embodiment 2
[0058] Step a) Disassemble a 35Ah lithium iron phosphate hard-shell battery whose capacity has decayed to 80%, cut off the tabs, and put the winding core (~600g) and residual electrolyte (~20g) into 1.5L of nitric acid with a concentration of 3M In the solution, stir and soak for 3 hours at 30°C.
[0059] The above-mentioned mixture is screened through a 100-mesh screen to remove insoluble impurities to obtain a suspension; the suspension is filtered under reduced pressure to obtain a recycled carbon source and the first liquid phase, and the pH value of the first liquid phase: pH (a ) = 0.8.
[0060] Step b) Add triethylamine dropwise to the above solution until pH (b)=1.5, filter to generate the first precipitate (~229g) whose main component is iron phosphate, the first lithium-containing solution.
[0061] Step c) Continue to add triethylamine solution dropwise to the first lithium-containing solution until pH (c)=5.0, and obtain the second lithium-containing solution afte...
Embodiment 3
[0067] Step a) Disassemble a 35Ah lithium iron phosphate hard-shell battery whose capacity has decayed to 80%, cut off the tabs, and put the winding core (~600g) and residual electrolyte (~20g) into 1.5L of 3M three Chloroacetic acid solution, then add 250ml 1M hydrogen peroxide solution, and stir and soak for 5h at 20°C.
[0068] Sieve the above mixture through a 100-mesh sieve to remove insoluble impurities to obtain a suspension; filter the suspension under reduced pressure to obtain a recovered carbon source and the first liquid phase. The pH of the first liquid phase is pH(a)=0.4.
[0069] Step b) Add triethylamine dropwise to the above solution until pH (b) = 1.0, and filter to generate the first precipitate (~220g) whose main component is iron phosphate, the first lithium-containing solution.
[0070] Step c) Continue to add triethylamine solution dropwise to the above solution until pH (c) = 7.0, and obtain the second lithium-containing solution after filtration.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com