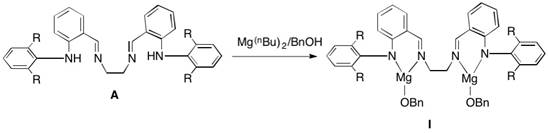
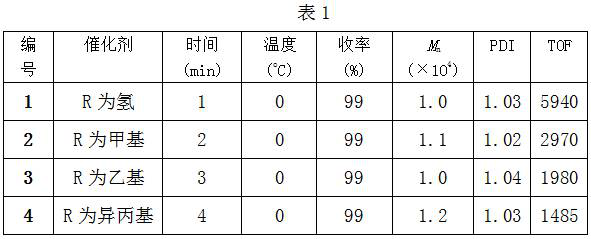
Utilize dinuclear amine imine magnesium complex to catalyze the method of caprolactone polymerization
A technology of binuclear amine imine magnesium and complexes, which is applied in the direction of magnesium organic compounds, can solve the problems of metal residues, etc., and achieve the effects of low cost, fast reaction rate, and various catalyst structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The structural formula of the ligand used is the above formula (A), where R is hydrogen, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol tetrahydrofuran solution (2.0 mol / L) to an equimolar amount of Mg ( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, 2.09 g of the ligand was dissolved in 20 mL of dry toluene, and added to Mg( n Bu) 2 In the reaction mixture with benzyl alcohol, after adding, the reaction liquid was naturally raised to room temperature, and then heated to 60 ° C for 3 hours. After the reaction was completed, the solvent was vacuum-dried, and the residue was washed with dry n-hexane, filtered, and then the product was collected and dried. Weighed to obtain 2.75 g of solid, yield 81.2%.
Embodiment 2
[0037] The structural formula of the ligand used is the above formula (A), where R is methyl, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol tetrahydrofuran solution (2.0 mol / L) to an equimolar amount at -10°C Mg( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, 2.37 g of the ligand was dissolved in 20 mL of dry toluene, and added to Mg( n Bu)2 and benzyl alcohol reaction mixture, after adding, the reaction liquid was naturally raised to room temperature, and then heated to 40 ° C for 12 hours, after the reaction was completed, the solvent was vacuum-dried, and the residue was washed with dry n-hexane, filtered, and then the product was collected and dried Weighed to obtain 3.27g solid, yield 89.1%.
Embodiment 3
[0039] The structural formula of the ligand used is the above formula (A), where R is ethyl, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol THF solution (2.0 mol / L) to an equimolar amount at -10°C Mg( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, 2.65 g of the ligand was dissolved in 30 mL of dry toluene, and added to Mg( n Bu) 2 In the reaction mixture with benzyl alcohol, after adding, the reaction liquid was naturally raised to room temperature, and then heated to 50 ° C for 4 hours. After the reaction was completed, the solvent was vacuum-dried, and the residue was washed with dry n-hexane, filtered, and then the product was collected and dried. Weighed to obtain 3.17g solid, yield 80.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com