A kind of synthetic method of aryl sulfinate compound
A technology of aryl sulfinate and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as difficulty, irritating odor, etc., and achieve the effect of reducing emission and high economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
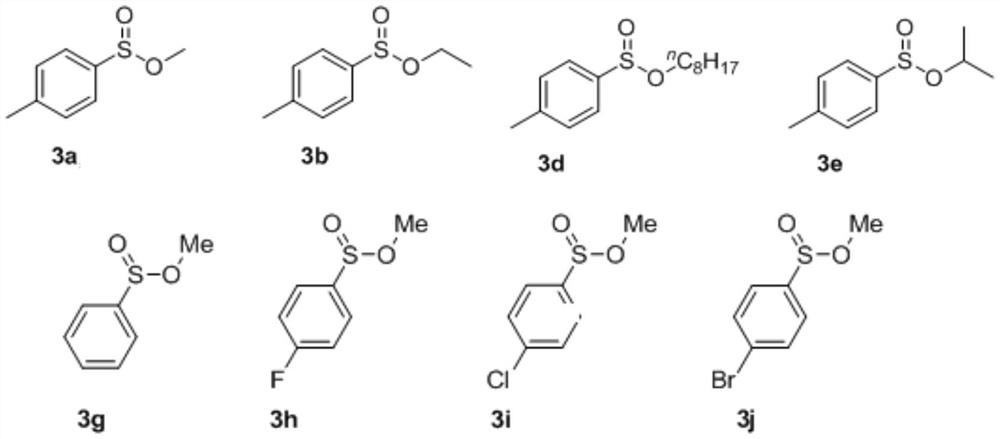
[0027] Under nitrogen protection, p-methylphenyltetrafluoroborate diazonium salt 1a (0.2 mmol), DABSO (0.2 mmol), methanol 2a (3 mL), cuprous iodide (10 mol%), azobisisobutyronitrile (AIBN, 10 mol%) was added to a Schlenk reaction tube and sealed. Heated to 80°C and the reaction time was over 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3a was obtained by column chromatography with a yield of 72%. 1 H NMR (400MHz, CDCl 3 ):δ7.57(d,J=8.0Hz,2H),7.32(d,J=8.0Hz,2H),3.44(s,3H), 2.41(s,3H). 13 C NMR (101MHz, CDCl 3 ): 142.8, 140.8, 129.7, 125.3, 49.3, 21.4.
Embodiment 2
[0029] Under nitrogen, p-methylphenyltetrafluoroborate diazonium salt 1a (0.2 mmol), DABSO (0.24 mmol), ethanol 2b (3 mL), cuprous bromide (10 mol%), benzoyl peroxide ( BPO, 5 mol%) was added to the Schlenk reaction tube and sealed. Heated to 80°C and the reaction time was over 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3b was obtained by column chromatography with a yield of 72%. 1 H NMR (400MHz, CDCl 3 ):δ7.58(d,J=8.0Hz,2H),7.32(d,J=8.0Hz,2H),4.04-4.12(m,1H),3.66-3.74(m,1H),2.41(s, 3H),1.26(t,J=7.1Hz,3H). 13 C NMR (101 MHz, CDCl 3 ): 142.6, 141.7, 129.6, 125.1, 60.7, 21.4, 15.5.
Embodiment 3
[0031] Under nitrogen protection, p-methylphenyltetrafluoroborate diazonium salt 1a (0.2 mmol), DABSO (0.1 mmol), n-butanol 2c (1 mL), cuprous chloride (10 mol%), azobisiso Nitrile (AIBN, 10 mol%) was added to the Schlenk reaction tube and sealed. Heated to 80°C and the reaction time was over 10 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3c was obtained by column chromatography in a yield of 63%. 1 H NMR (400MHz, CDCl 3 ): δ7.59(d, J=8.0Hz, 2H), 7.33(d, J=8.0Hz, 2H), 3.94-4.00(m, 1H), 3.53-3.59(m, 1H), 2.42(s, 3H), 1.61-1.67(m, 2H), 0.90(t, J=7.4Hz, 3H). 13 C NMR (101MHz, CDCl 3 ): 142.6, 141.8, 129.7, 125.2, 66.1, 23.1, 21.5, 10.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com