Preparation method of 2,5-dihydrofuran
A technology of dicyanofuran and furandicarboxaldehyde, applied in the direction of organic chemistry and the like, can solve the problems such as the difficulty of synthesizing aromatic heterocyclic nitrile compounds, and achieve the effects of avoiding the generation of polymerization by-products, the reaction process is efficient, and the product purity is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: 2mmol 2,5-furandicarbaldehyde, 3.5mL 1.5mol / L aqueous hydroxylamine solution, and 10mL water were added to a 25mL round bottom flask. Stir and heat up to 100°C, react for 6h. When finished, cool to room temperature. The 2,5-furandicarbaldehyde dioxime product was obtained by filtration, and the separation yield was 95%. Add 1 mmol of 2,5-furandicarbaldehyde dioxime, 30 mg of MgO, and 8 mL of N,N-dimethylformamide into a 25 mL round bottom flask. Stir and heat up to 150°C, react for 1h. When finished, cool to room temperature. After evaporating the solvent from the reaction liquid, a white solid of 2,5-dicyanofuran was obtained by column chromatography separation, and the separation yield was 96%.
Embodiment 2
[0018] Example 2: 2mmol 2,5-furandicarbaldehyde, 3.5mL 1.5mol / L aqueous solution of hydroxylamine, and 10mL methanol were added to a 25mL round bottom flask. Stir and heat up to 60°C, react for 14h. When finished, cool to room temperature. The 2,5-furandicarbaldehyde dioxime product was obtained by filtration, and the separation yield was 97%. Then 1mmol 2,5-furandicarbaldehyde dioxime, 30mg CeO 2 , 8mL o-xylene was added to a 25mL round bottom flask. Stir and heat up to 140°C, react for 3h. When finished, cool to room temperature. After evaporating the solvent from the reaction liquid, a white solid of 2,5-dicyanofuran was obtained by column chromatography separation, and the separation yield was 98%.
Embodiment 3
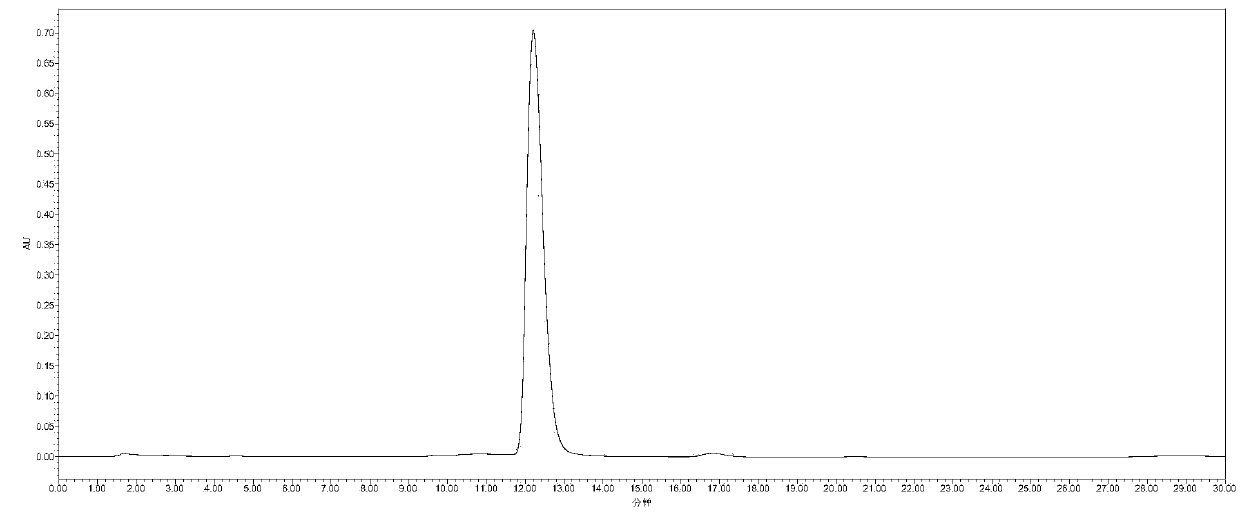
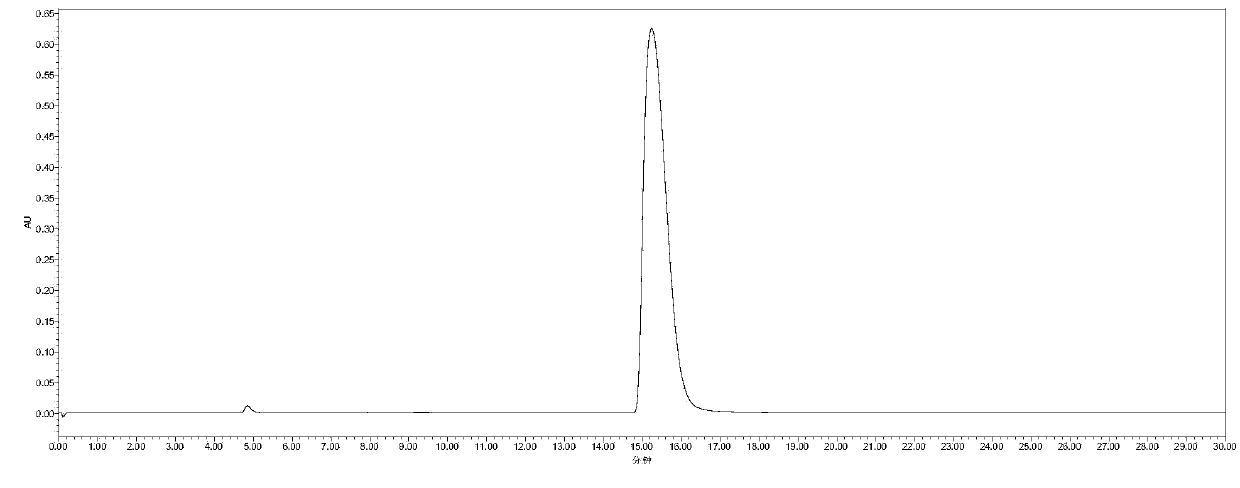
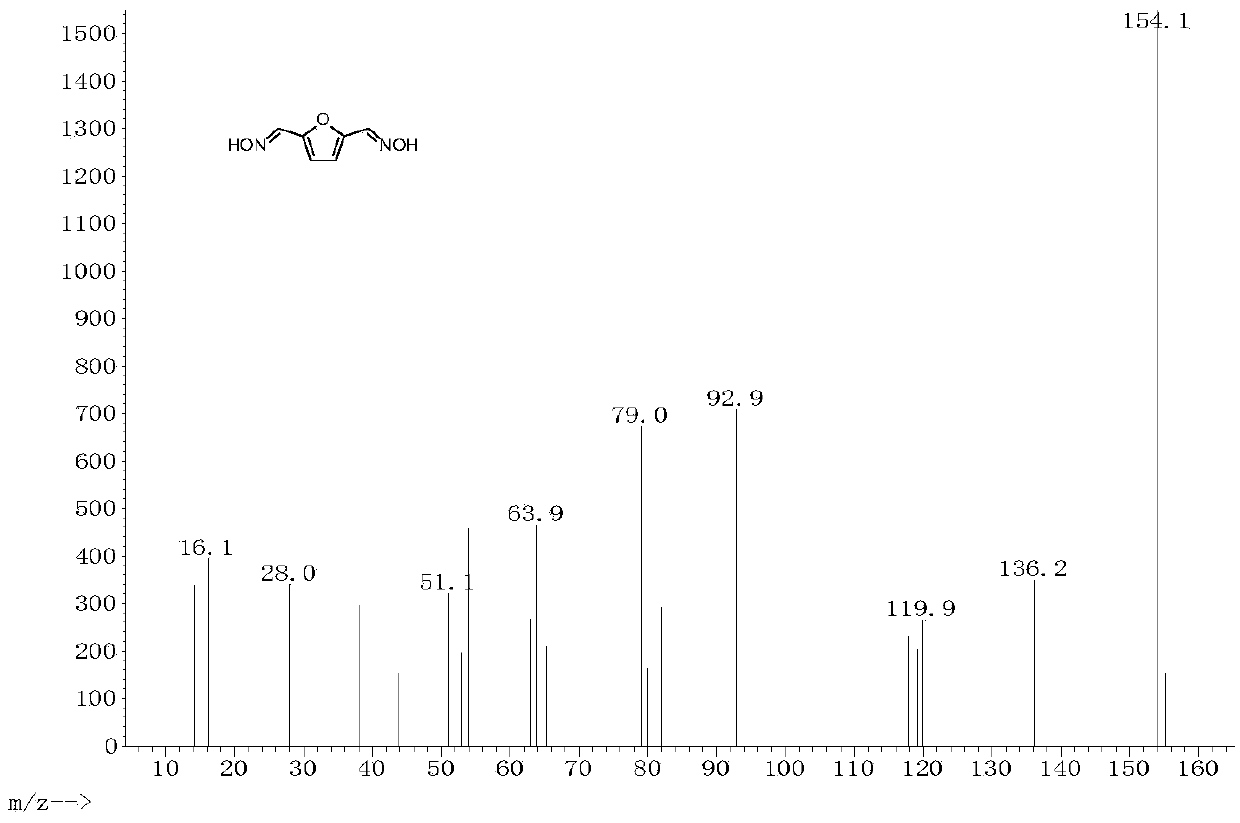
[0019] Example 3: 2mmol 2,5-furandicarbaldehyde, 3.5mL 1.5mol / L aqueous hydroxylamine solution, and 10mL ethanol were added to a 25mL round bottom flask. Stir and heat up to 80°C, react for 12h. When finished, cool to room temperature. The 2,5-furandicarbaldehyde dioxime product was obtained by filtration, the separation yield was 99%, and the HPLC and GC-MS spectra were shown in figure 1 and image 3 . Then add 1 mmol 2,5-furandicarbaldehyde dioxime, 50 mg α-MnO 2 , 8mL of toluene was added to a 25mL round bottom flask. Stir and heat up to 110°C, react for 8h. When finished, cool to room temperature. After evaporating the solvent from the reaction solution, the column chromatography separation method was used to obtain 2,5-dicyanofuran as a white solid with a separation yield of 99%. The HPLC spectrum and GC-MS spectrum are shown in figure 2 and Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com