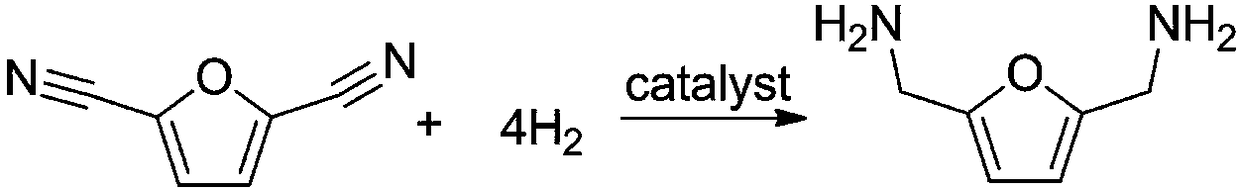
Method for synthesizing 2,5-dimethylamino furan through catalytic hydrogenation of 2,5-dicyanofuran
A technology of dimethylaminofuran and dicyanofuran, which is applied in the field of catalytic hydrogenation of 2,5-dicyanofuran to synthesize 2,5-dimethylaminofuran, can solve problems such as difficulty in repeated use, and achieve easy Separation, good application prospects, and good product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
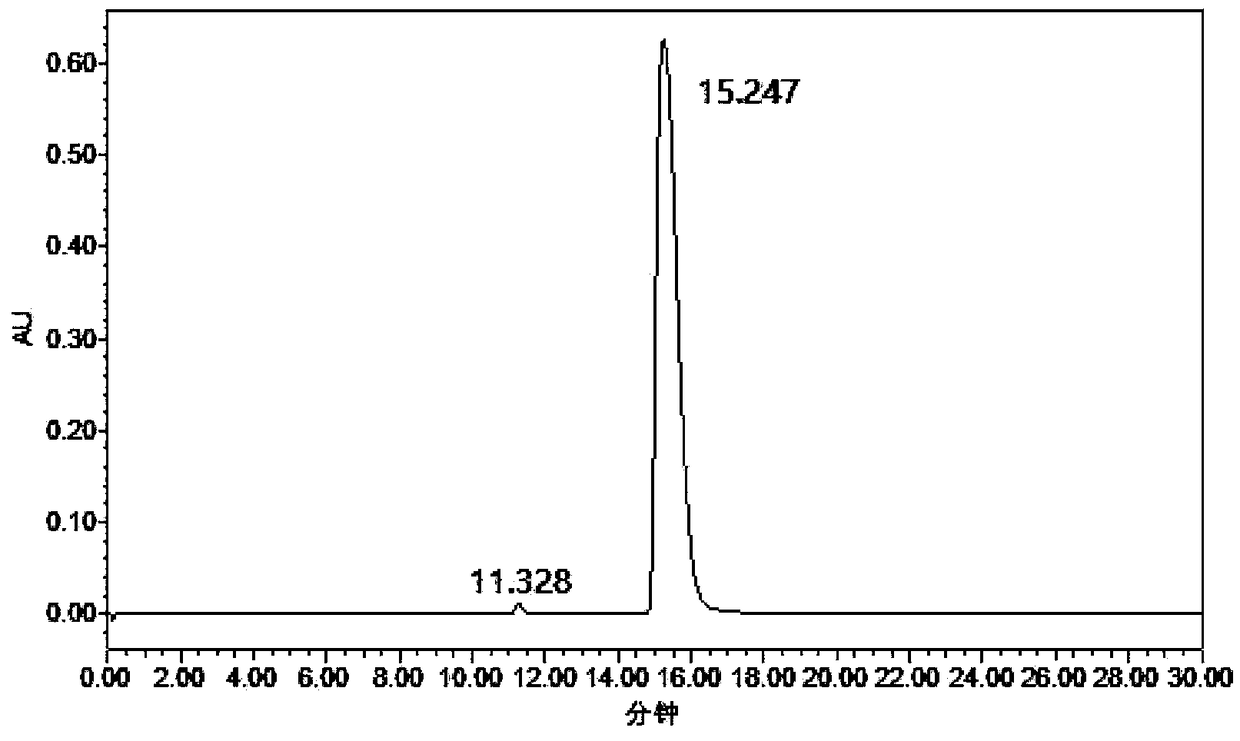
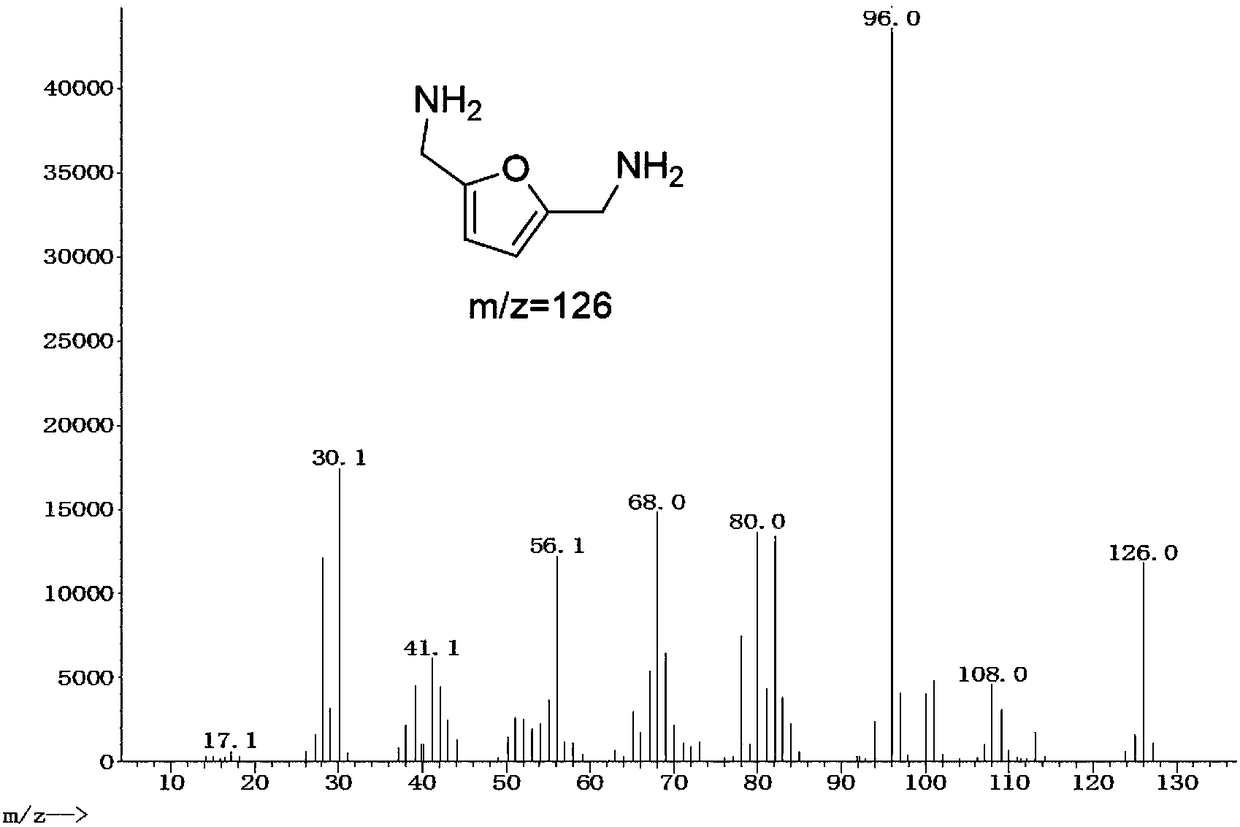
[0018] Example: 1 mmol of 2,5-dicyanofuran, 30 mg of Rh / HZSM-5 catalyst with a loading capacity of 5 wt %, and 7 mL of methanol were added to a 15 mL reactor, and the air in the reactor was replaced with hydrogen for 10 times. After raising the temperature to 120°C, fill it with hydrogen to 2.5MPa, and start stirring. If the partial pressure of hydrogen drops, make up to 2.5MPa. After 3 hours of reaction, it was cooled to room temperature. The reaction solution was centrifuged to separate the catalyst, and samples were taken, and liquid chromatography was used for product analysis. Such as figure 1 Shown is the high-performance liquid chromatography (HPLC) figure of reaction solution, wherein the retention time is 11.328min is the raw material 2,5-dicyanofuran, and the retention time is 15.247min is the product 2,5-dimethylaminofuran . A small amount of by-products are diamine and triamine compounds containing multiple furan rings. Using the external standard method as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com