Preparation method of carboxylic ester compounds
An ester compound and compound technology, applied in the field of compound synthesis, can solve the problems of low catalyst activity, reduced production capacity, difficult to handle, etc., and achieve the effects of high catalyst activity, reduced environmental protection pressure, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of carboxylate compound, comprises the following steps:
[0027] a) Under the action of a catalyst, the compound of the formula (A) and the compound of the formula (B) react to obtain a carboxylate compound of the structure shown in the formula (C);
[0028]
[0029]
[0030] In formula (A)~(C), R 1 from C 1 ~C 2 Alkyl, R 2 ~R 5 independently selected from hydrogen, halogen, C 1 ~C 3 Alkyl or C 1 ~C 3 Haloalkyl;
[0031] The catalysts include azobisisobutyronitrile and / or peroxygen catalysts.
[0032] In the preparation method provided by the present invention, the structural compound of formula (A) and the structural compound of formula (B) are used as reaction raw materials. Wherein, R in the compound of the formula (A) structure 1 The group is a methyl group or an ethyl group, that is, the compound of the formula (A) can specifically be methyl pyritinate or ethyl pyritinate; R in the compound of the...
Embodiment 1
[0045] Carboxylate compounds are synthesized according to the following chemical equation:
[0046]
[0047] The specific process includes:
[0048] 1) Under the protection of nitrogen, add 145.1g methyl peroxyneodecanoate to a 500ml autoclave, close the autoclave, heat up to 60°C, pump 0.71g tert-butyl peroxyneodecanoate and 283.4g trifluoro The trichloroethane mixture was pumped in after 2 hours. Continue to keep warm for 4h.
[0049] 2) Cool down to 30°C, transfer the reaction solution to a four-necked flask, and recover excess trifluorotrichloroethane under reduced pressure to obtain 330.2 g of the product, which was analyzed by GC with a product content of 99.0% and a yield of 99.2%.
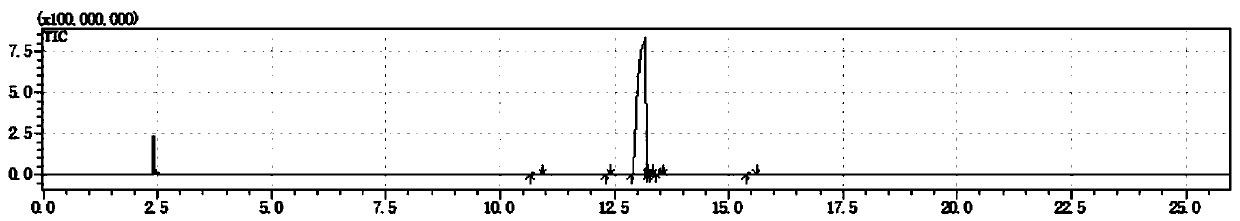
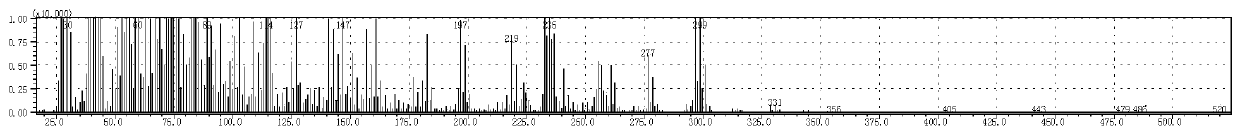
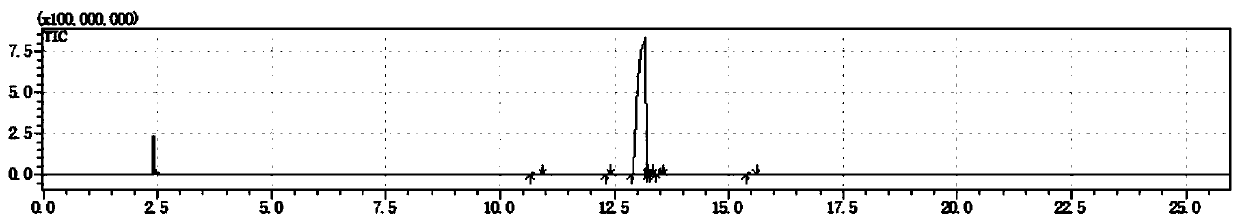
[0050] Gas chromatography and mass spectrometry are carried out to the product prepared in this embodiment, wherein the chromatographic analysis results are as follows: figure 1 and as shown in Table 1, figure 1 It is the chromatogram that the embodiment of the present invention 1 pr...
Embodiment 2
[0054] Carboxylate compounds are synthesized according to the following chemical equation:
[0055]
[0056] The specific process includes:
[0057] 1) Under the protection of nitrogen, add 156.2g of ethyl bentinate to a 500ml autoclave, close the autoclave, heat up to 40°C, pump 2.84g of azobisisobutyronitrile and 169.5g of dichloromethane through the feeding port to mix liquid, pumping ends after 2h. Continue to keep warm for 4h.
[0058] 2) Cool down to 30° C., transfer the reaction solution to a four-neck flask, and recover excess methylene chloride under reduced pressure to obtain 241.2 g of product. GC analysis shows that the product content is 98.0%, and the yield is 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com