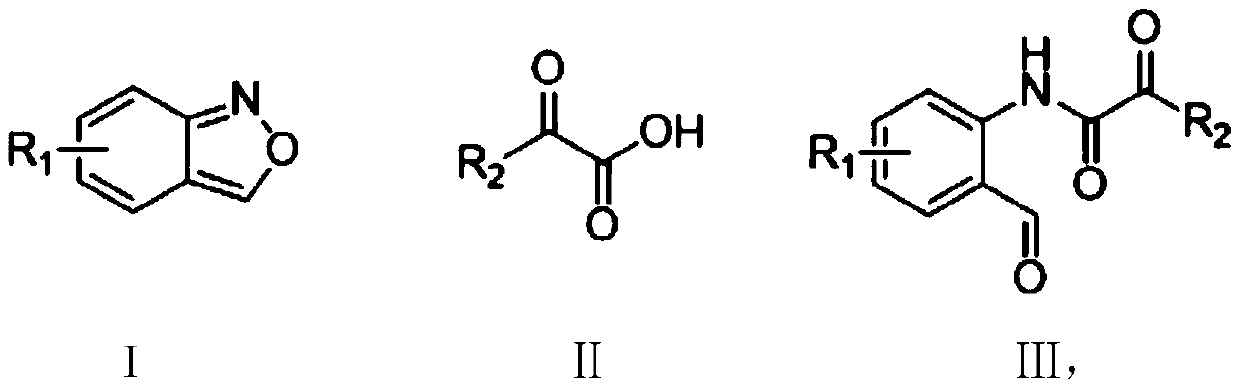
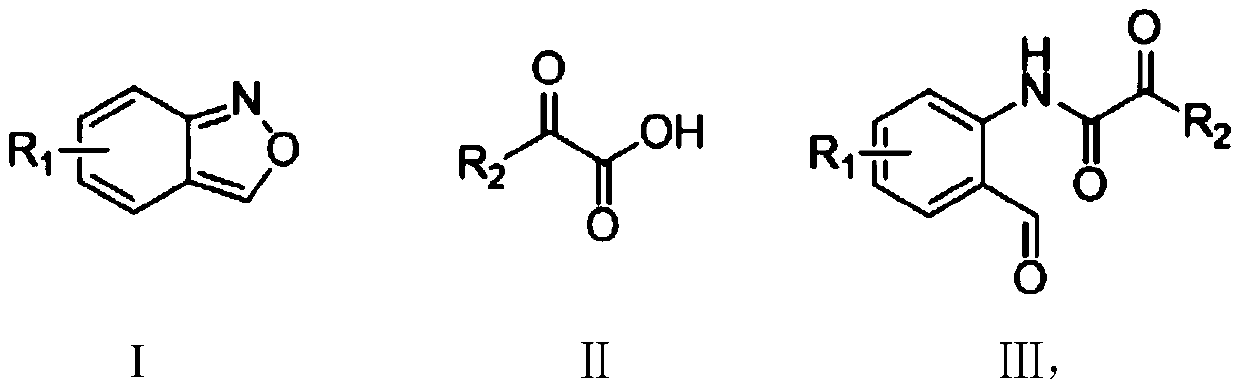
Method for synthesizing ortho-aldehyde group-containing alpha-ketoamide compound
A synthesis method and ketoamide technology are applied in the preparation of organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. Expensive, increase synthesis cost and other problems, to achieve the effect of wide application range of substrate, cheap dosage, increase synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
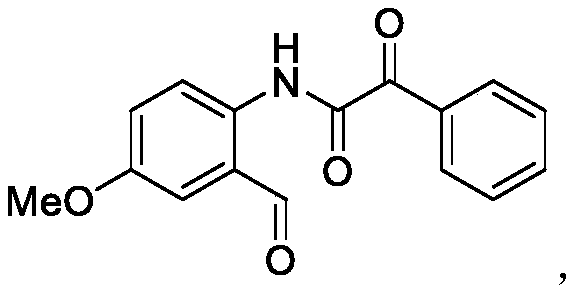
[0032] The structural formula of the ortho aldehyde group α-ketoamide compound prepared in this embodiment is as follows:
[0033]
[0034] The preparation method is: 0.3mmol 5-methoxybenzo[c]isoxazole (44.7mg), 0.6mmol benzoylformic acid (90.1mg), 0.015mmol copper bromide (3.3mg) and 0.06mmol triphenyl Phosphine (15.7 mg) was added to a 25 ml schlenk tube, and the reaction tube was replaced with argon three times under reduced pressure. 1,2-Dichloroethane (3 ml) was added, and stirred at 110° C. for 12 hours. After the reaction is completed, add 100-200 mesh column chromatography silica gel, and distill the solvent under reduced pressure. Elution, by means of TLC elution tracking detection, collect the eluate containing the target product, combine the eluate of the target product, evaporate and concentrate to obtain the α-ketoamide compound of the ortho aldehyde group shown in formula III. The material was a yellow solid, 72% yield.
[0035] Characterization data: m.p.1...
Embodiment 2
[0037] The structural formula of the ortho aldehyde group α-ketoamide compound prepared in this embodiment is as follows:
[0038]
[0039] The preparation method is: 0.3mmol 5-fluorobenzo [c] isoxazole (41.1mg), 0.6mmol benzoylformic acid (90.1mg), 0.015mmol CuBr (PPh 3 ) 3(13.9 mg) was added to a 25 ml schlenk tube, and the reaction tube was replaced with argon three times under reduced pressure. 1,2-Dichloroethane (3 ml) was added, and stirred at 110° C. for 12 hours. After the reaction is completed, add 100-200 mesh silica gel for column chromatography, and distill the solvent under reduced pressure. Elution, by means of TLC elution tracking detection, collect the eluate containing the target product, combine the eluate of the target product, evaporate and concentrate to obtain the α-ketoamide compound of the ortho aldehyde group shown in formula III. The material was a yellow solid in 70% yield.
[0040] Characterization data: m.p.133-135℃; 1 H NMR (400MHz, CDCl ...
Embodiment 3
[0042] The structural formula of the ortho aldehyde group α-ketoamide compound prepared in this embodiment is as follows:
[0043]
[0044] The preparation method is:
[0045] 0.3 mmol 5-chlorobenzo[c]isoxazole (46 mg), 0.6 mmol benzoylformic acid (90.1 mg), 0.015 mmol copper bromide (3.3 mg) and 0.06 mmol triphenylphosphine (15.7 mg) were added Into a 25 ml schlenk tube, the reaction tube was replaced with argon three times under reduced pressure. 1,2-Dichloroethane (3 ml) was added, and stirred at 110° C. for 12 hours. After the reaction is completed, add 100-200 mesh silica gel for column chromatography, and distill the solvent under reduced pressure. Elution, by means of TLC elution tracking detection, collect the eluate containing the target product, combine the eluate of the target product, evaporate and concentrate to obtain the α-ketoamide compound of the ortho aldehyde group shown in formula III. The material was a yellow solid in 75% yield.
[0046] Characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com