Preparation method of Stendra
A technology of avanafil and its compounds, which is applied in the field of preparation of avanafil, can solve the problems of expensive, excessive, and difficult activation reagents, and achieve the benefits of large-scale industrial production, reduction of preparation costs, and reduction of manpower. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
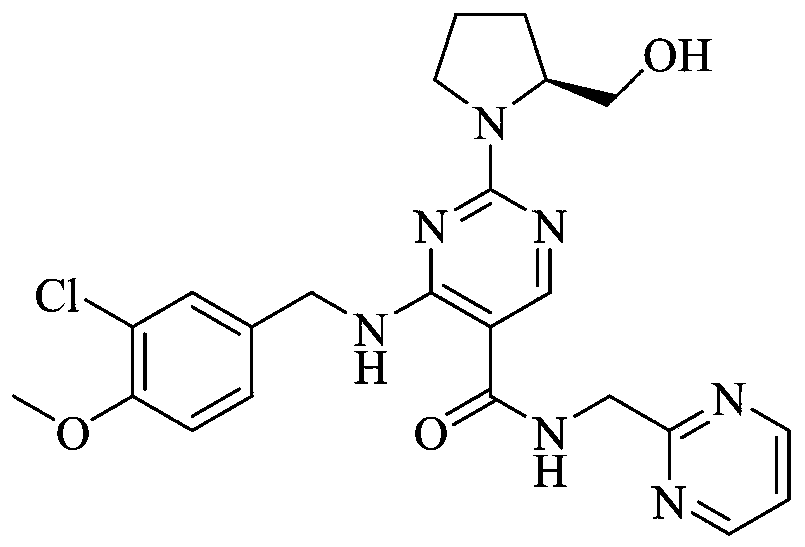
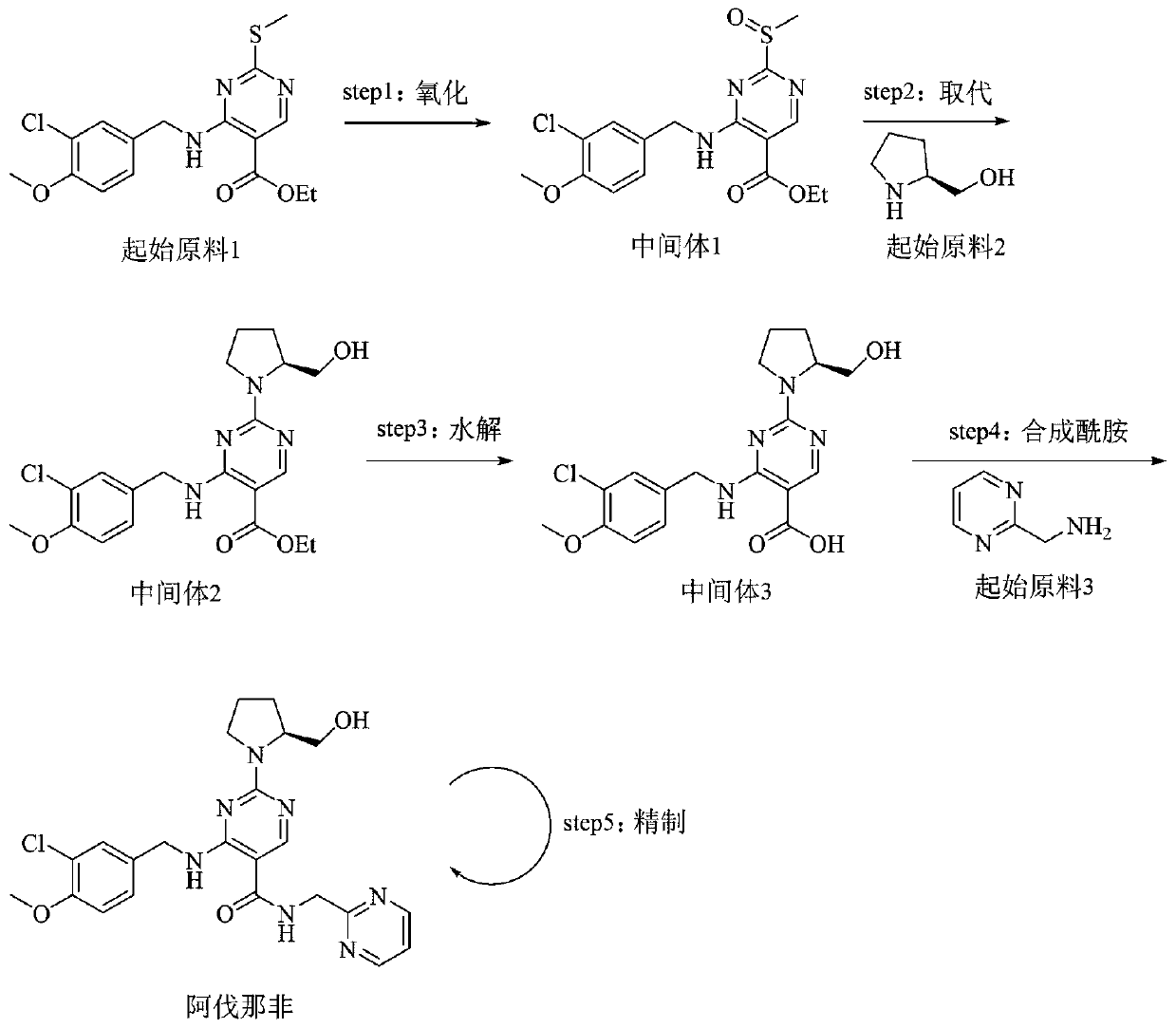
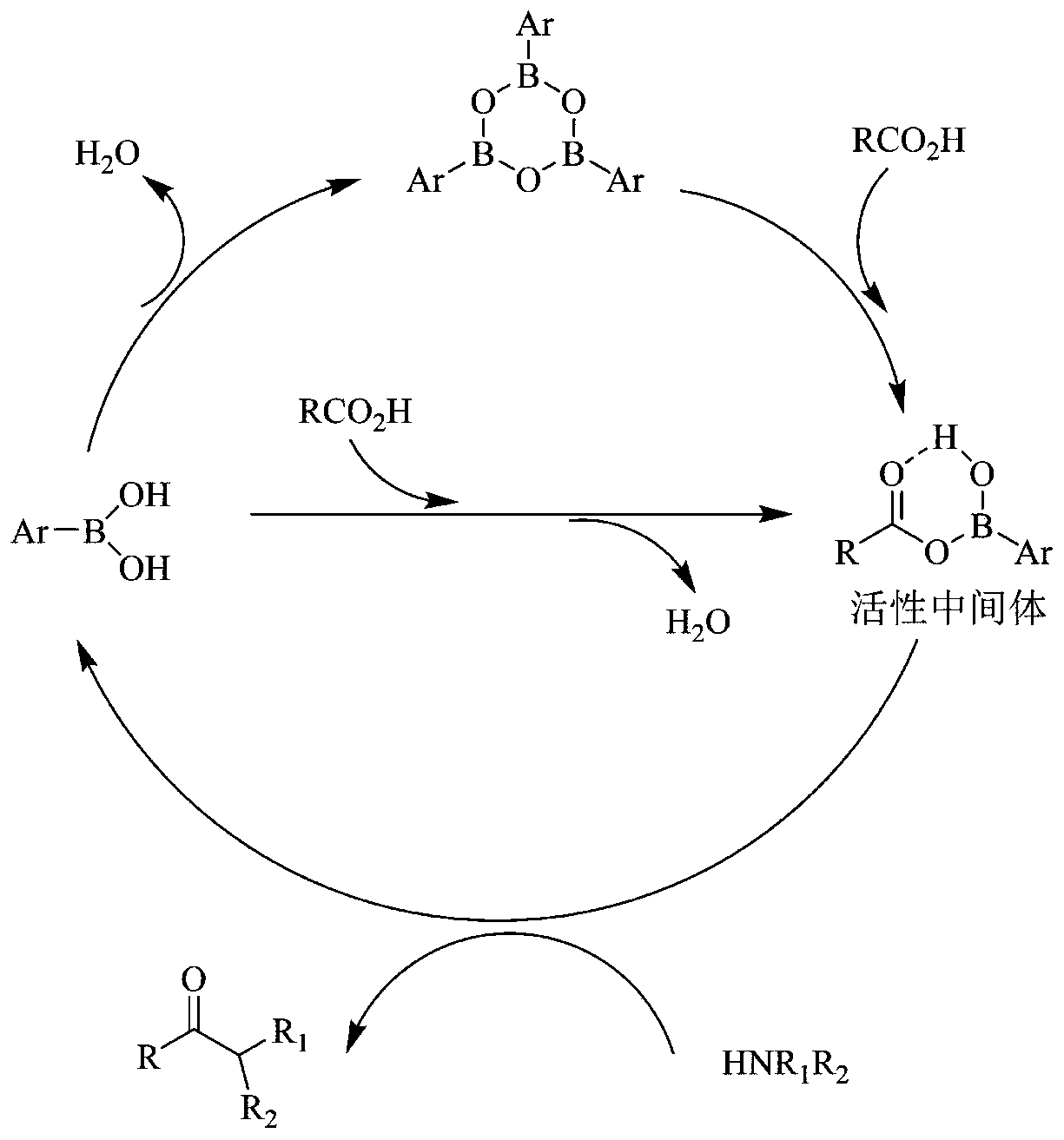
[0033] 4-[(3-Chloro-4-methoxyphenyl)methylamino]-2-[(S)-2-hydroxymethylpyrrol-1-yl]pyrimidine-5-carboxylic acid (39.3g, 100mmol , 1.0eq), 2-aminomethylpyrimidine (10.9g, 100mmol, 1.0eq) and porphyrin borate (0.79g, 1mmol, 0.01eq) were added to 300mL toluene solution, heated and stirred to reflux state, and reacted for 8 hours.
[0034] After the reaction, the temperature of the reaction solution was slowly lowered to 10-20° C., and 300 mL of 5 wt % hydrochloric acid aqueous solution was added dropwise. Control the temperature of the system not to exceed 20°C, stir for 30 minutes, and recover the boric acid porphyrin catalyst by filtration. Separation and take the lower aqueous phase, add 300mL dichloromethane to extract and wash the aqueous phase, slowly add solid NaOH to adjust the pH of the aqueous phase to 7.0, stir and crystallize at room temperature for 2 hours, filter to obtain the crude product of avanafil, wash the filter cake twice with purified water .
[0035] Add...
Embodiment 2
[0037] 4-[(3-Chloro-4-methoxyphenyl)methylamino]-2-[(S)-2-hydroxymethylpyrrol-1-yl]pyrimidine-5-carboxylic acid (39.3g, 100mmol , 1.0eq), 2-aminomethylpyrimidine (10.9g, 100mmol, 1.0eq) and boric acid porphyrin (3.95g, 5mmol, 0.05eq) were added to 400mL toluene solution, heated and stirred to reflux state, and reacted for 12 hours.
[0038] After the reaction, the temperature of the reaction solution was slowly lowered to 10-20° C., and 200 mL of 8 wt % hydrochloric acid aqueous solution was added dropwise. Control the temperature of the system not to exceed 20°C, stir for 30 minutes, and recover the boric acid porphyrin catalyst by filtration. Separation and take the lower aqueous phase, add 200mL dichloromethane to extract and wash the aqueous phase, slowly add solid NaOH to adjust the pH of the aqueous phase to 7.0, stir and crystallize at room temperature for 2 hours, filter to obtain the crude product of avanafil, wash the filter cake twice with purified water .
[0039...
Embodiment 3
[0041] 4-[(3-Chloro-4-methoxyphenyl)methylamino]-2-[(S)-2-hydroxymethylpyrrol-1-yl]pyrimidine-5-carboxylic acid (39.3g, 100mmol , 1.0eq), 2-aminomethylpyrimidine (12.0g, 110mmol, 1.1eq) and boric acid porphyrin (3.95g, 5mmol, 0.05eq) were added to 400mL toluene solution, heated and stirred to reflux state, and reacted for 12 hours.
[0042]After the reaction, the temperature of the reaction solution was slowly lowered to 10-20° C., and 400 mL of 5 wt % hydrochloric acid aqueous solution was added dropwise. Control the temperature of the system not to exceed 20°C, stir for 30 minutes, and recover the boric acid porphyrin catalyst by filtration. Separation and take the lower aqueous phase, add 400mL dichloromethane to extract and wash the aqueous phase, slowly add solid NaOH to adjust the pH of the aqueous phase to 7.0, stir and crystallize at room temperature for 2 hours, filter to obtain the crude product of avanafil, wash the filter cake twice with purified water .
[0043]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com