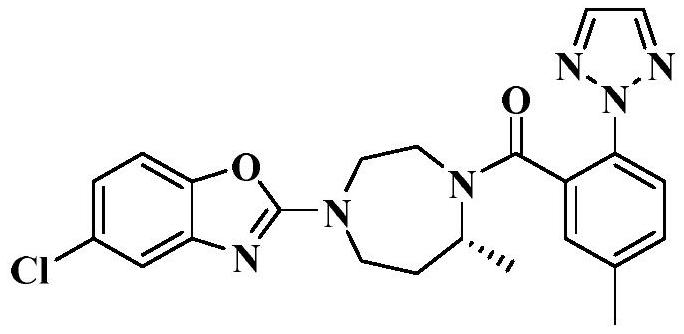
A kind of preparation method of Suwo Leisheng intermediate
An intermediate and time technology, applied in the field of medicine, can solve the problems that the product does not meet the quality standard of pharmaceutical intermediates, the catalyst cuprous iodide is difficult to remove, and the content of isomer compound 2 is high, so as to achieve high yield and product purity , Improve labor protection and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
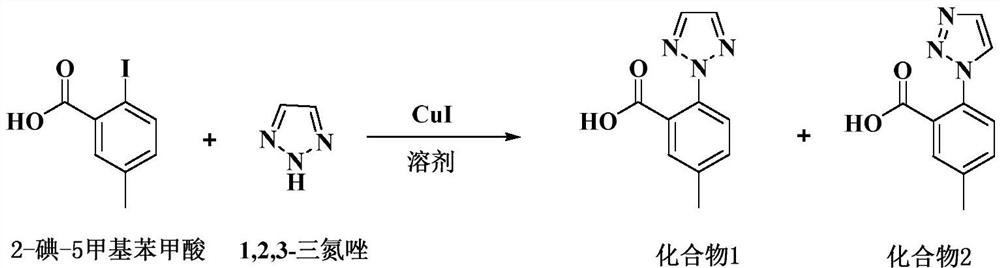
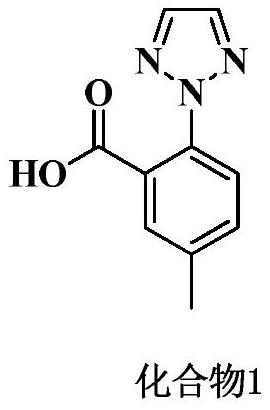
[0028] Add 66g of acetone and 8.4g of 2-iodo-5-methylbenzoic acid into a 250ML reaction flask, stir to dissolve, add 8.9g of anhydrous potassium carbonate, heat up to reflux (55-60°C) for half an hour, then add 2.9g of 1, 2,3-triazole, 0.061g cuprous iodide. Insulate and reflux (55-60°C) for 8 hours, take samples, and take samples every 1h for testing. After the reaction is completed, cool to room temperature and filter. The filter cake is washed twice with 3g of acetone to obtain a white solid. At room temperature (20-30°C) The white solid was dissolved in 84 g of purified water and stirred for 30 min, and hydrochloric acid was added dropwise at room temperature to adjust the pH of the system to 1.5-2.0 to obtain a large amount of white solid. Filter and wash the filter cake twice with purified water. The obtained solid was dried to dryness at 50±5°C to obtain a crude compound 1.
[0029] Add the crude compound 1 obtained in the previous step, 10g of absolute ethanol and 10...
Embodiment 2
[0031] Add 1.1kg of acetone and 131g of 2-iodo-5-methylbenzoic acid into a 5L reaction flask, stir to dissolve, add 350g of anhydrous potassium carbonate, heat up to reflux (55-60°C) for half an hour, then add 114g of 1,2, 3-triazole, 2.4g cuprous iodide. Insulate and reflux (55-60°C) for 8 hours, take samples, and take samples every 1h for detection. After the reaction is completed, cool to room temperature and filter. The filter cake is washed twice with 120g of acetone to obtain a white solid. At room temperature (20-30°C) The white solid was dissolved in 1.3 kg of purified water and stirred for 30 min, and hydrochloric acid was added dropwise at room temperature to adjust the pH of the system to 1.5-2.0 to obtain a large amount of white solid. Stir for 1-2 hours, filter, and wash the filter cake twice with purified water. The obtained solid was dried to dryness at 50±5°C to obtain a crude compound 1.
[0032] Add crude compound 1, 0.2kg of absolute ethanol and 0kg of pur...
Embodiment 3
[0034]Add 2.5kg of acetone and 314g of 2-iodo-5-methylbenzoic acid into a 5L reaction flask, stir to dissolve, add 332g of anhydrous potassium carbonate, heat up to reflux (55-60°C) for half an hour, then add 108g of 1,2, 3-triazole, 2.28g cuprous iodide. Insulate and reflux (55-60°C) for 8 hours, take samples, and take samples every 1h for detection. After the reaction is completed, cool to room temperature and filter. The filter cake is washed twice with 250g acetone to obtain a white solid. At room temperature (20-30°C) The white solid was dissolved in 3.1 kg of purified water and stirred for 30 min, and hydrochloric acid was added dropwise at room temperature to adjust the pH of the system to 1.5-2.0 to obtain a large amount of white solid. Stir for 1-2 hours, filter, and wash the filter cake twice with purified water. The obtained solid was dried to dryness at 50±5°C to obtain a crude compound 1.
[0035] Add crude compound 1, 0.5kg of absolute ethanol and 0.5kg of puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com