Electrolyte material and preparation method thereof
An electrolyte material, high conductivity technology, applied in circuits, fuel cells, electrical components, etc., can solve the shortage of materials with high proton or oxygen ion conductivity, limit the operating temperature of fuel cells, and have great difficulties in commercialization, etc. problem, to achieve the effect of improving carbon monoxide tolerance, improving toxicity, improving performance and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Will NH 4 HCO 3 Dissolve in deionized water to form a transparent solution; then drop the above transparent solution into 0.1mol / L H 3 PW 12 o 40 In solution, NH in raw material 4 HCO 3 / H 3 PW 12 o 40 / H 3 PO 4 The molar ratio is 3:1:0, stir vigorously to obtain a uniform mixture, heat the mixture to 200°C and evaporate the excess water; the obtained white powder is dried at 100°C for 13 hours.
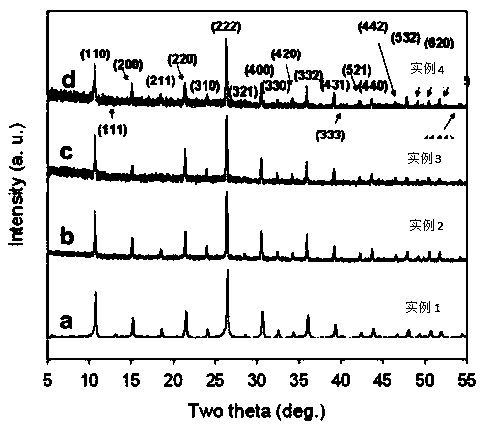
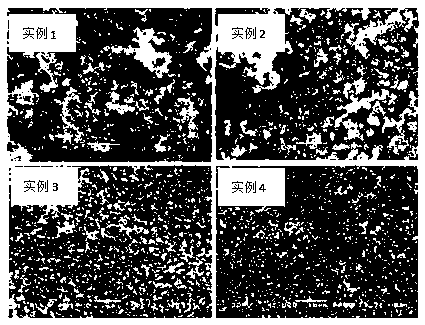
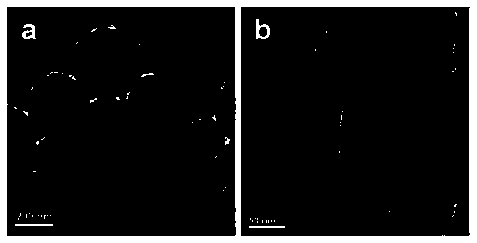
[0036] The sample was subjected to XRD test and electron microscope scanning, and the obtained results were as follows: figure 1 , figure 2As shown, from which we can see that the obtained sample is a symmetric cubic single phase with a spherical microstructure, from Figure 4 It can be seen that the conductivity is lower.
Embodiment 2
[0038] The difference from the above examples is that the NH 4 HCO 3 Dissolve in deionized water to form a transparent solution; then drop the above transparent solution into 0.1mol / L H 3 PW 12 o 40 In solution, NH in raw material 4 HCO 3 / H 3 PW 12 o 40 / H 3 PO 4 The molar ratio is 3:1:3, stir vigorously to obtain a uniform mixture, heat to 200°C to evaporate excess water; the obtained white powder is dried at 110°C for 12 hours.
[0039] The sample was subjected to XRD test and electron microscope scanning, and the obtained results were as follows: figure 1 , figure 2 As shown, from which we can see that the obtained sample is a symmetrical cubic single phase, which is shifted to a small angle, indicating that the unit cell is enlarged, phosphoric acid is successfully intercalated, and has a spherical microstructure. from Figure 5 It can be seen that the conductivity is quite different from Example 1.
Embodiment 3
[0041] The difference from the above examples is that the NH 4 HCO 3 Dissolve in deionized water to form a transparent solution; then drop the above transparent solution into 0.1mol / L H 3 PW 12 o 40 In solution, NH in raw material 4 HCO 3 / H 3 PW 12 o 40 / H 3 PO 4 The molar ratio is 3:1:5, stir vigorously to obtain a uniform mixture, heat to 200°C and evaporate the excess water; the obtained white powder is dried at 120°C for 11 hours.
[0042] The sample was subjected to XRD test and electron microscope scanning, and the obtained results were as follows: figure 1 , figure 2 As shown, we can see from it that the obtained sample is a symmetrical cubic single phase, and there is basically no shift compared with Example 2, indicating that phosphoric acid cannot continue to enter the unit cell, but hangs outside the unit cell, with a spherical microstructure. from Figure 5 It can be seen that the conductivity is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com