Synthesis method of 3-bromoquinoline compound
A synthesis method and technology for aniline compounds, applied in the direction of organic chemistry and the like, can solve the problems of incapable of industrialized production, unobtainable raw materials, difficult separation, etc., and achieve the effects of few reaction steps, cheap raw materials, and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
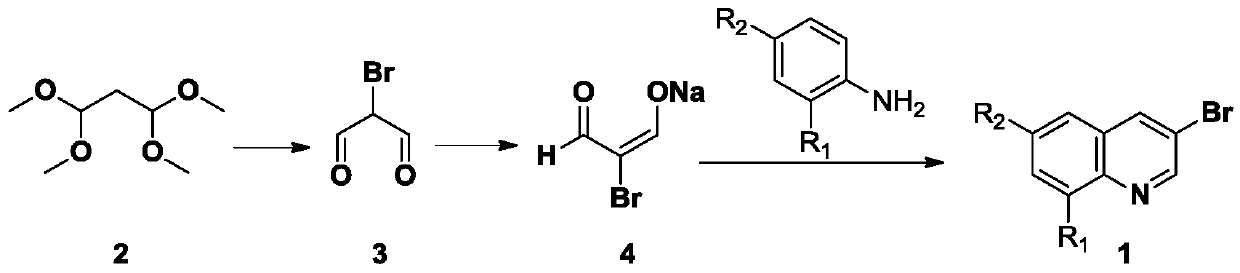
[0025] Embodiment 1: the synthesis of 3-bromoquinoline
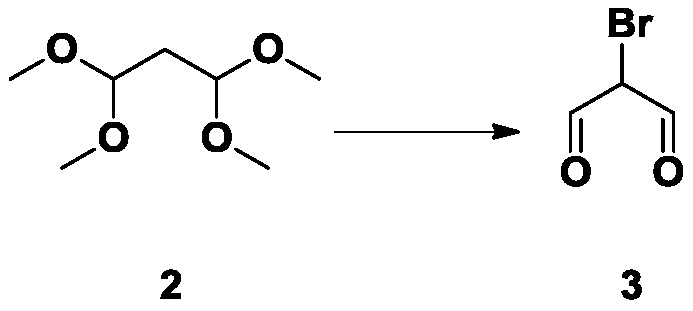
[0026] 1. Synthesis of Intermediate 3
[0027] Add 100g of compound 1 to 96ml of water, slowly add 5.4ml of hydrochloric acid and 105.6g of liquid bromine dropwise at 0°C, stir overnight at room temperature, spin off the water at 45°C, filter, and wash the solid with PE:EA times, dried to obtain 50g intermediate 3.
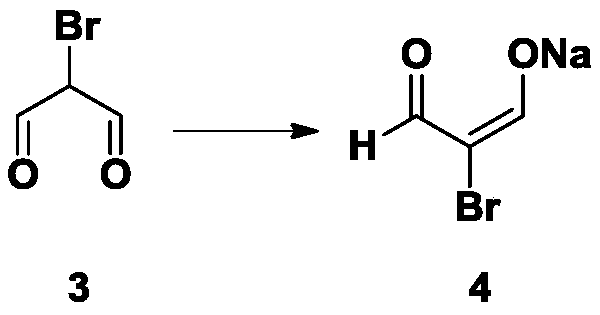
[0028] 2. Synthesis of Intermediate 4
[0029] Add 16.8g NaOH to 400ml of water, then slowly add 60g of intermediate 3, spin off the water under reduced pressure until solids precipitate, then add 1L of cold acetone, stir overnight at 4 degrees, filter, and dissolve the solids in 500ml of cold water The acetone was washed twice, and the solid was pulled dry with an oil pump to obtain 68g of intermediate 4.
[0030] 3. Synthesis of 3-bromoquinoline 1
[0031] Add 100g of intermediate 3 and 53.8g of anthranilic acid to 500ml of ethanol, then slowly add 150ml of 30% hydrobromic acetic acid dropwise, heat to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com