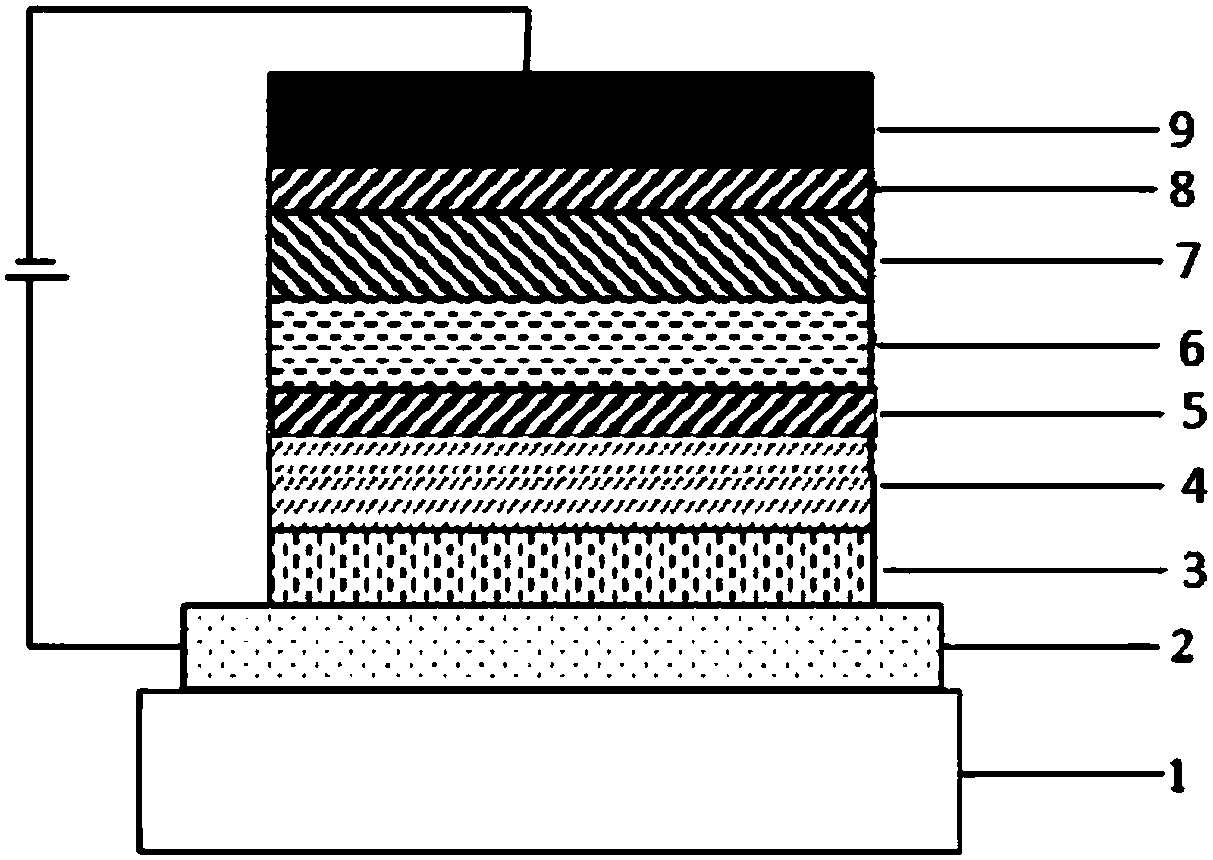
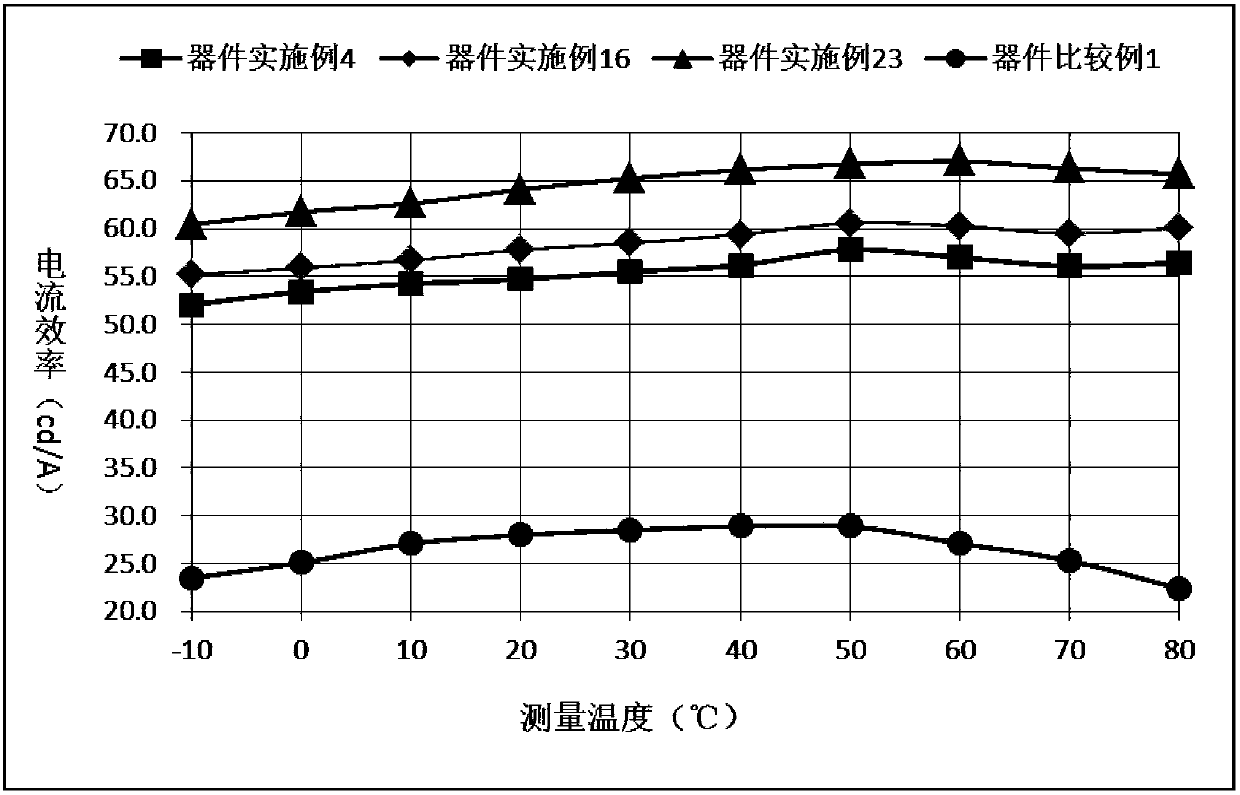
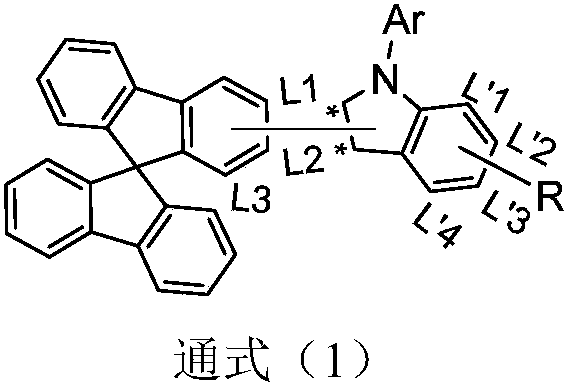
Spirofluorene derivative type organic compound and application thereof to organic light-emitting diodes
A technology of electroluminescent devices and organic compounds, which is applied in the field of spirofluorene derivative organic compounds and their application in organic electroluminescent devices, and can solve the problems of different performances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the synthesis of intermediate II and intermediate VI
[0066] a. Synthesis of Intermediate II
[0067]
[0068] (1) Under the protection of nitrogen, weigh raw material A and dissolve it in tetrahydrofuran, cool to -78°C, then add 1.6mol / L tetrahydrofuran solution of n-butyllithium to the reaction system, react at -78°C for 3h, then add Triisopropyl borate, react for 2 hours, then raise the reaction system to 0°C, add 2mol / L hydrochloric acid solution, stir for 3 hours, the reaction is complete, add ether for extraction, add anhydrous magnesium sulfate to the extract to dry, rotary evaporate, use ethanol solvent recrystallization to obtain intermediate B; the molar ratio of raw material A to n-butyllithium is 1:1-1.5; the molar ratio of intermediate S4 to triisopropyl borate is 1:1-1.5.
[0069] (2) Under nitrogen protection, weigh intermediate B, Fe(NO 3 ) 3 .9H 2 O, dissolved in toluene; heated to 90-110°C, reacted for 10-24 hours, took a sample p...
Embodiment 2
[0102] Embodiment 2: the synthesis of compound 5:
[0103]
[0104] (1) In a 250ml three-neck flask, under the protection of nitrogen, add 0.05mol raw material I-1, 0.075mol intermediate II-1, dissolve with a mixed solvent (90ml toluene, 45ml ethanol), and then add 0.15mol Na 2 CO 3 aqueous solution (2M), stirred under nitrogen for 1 hour, then added 0.0005mol Pd(PPh 3 ) 4 , heating to reflux for 15 hours, sampling point plate, the reaction is complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain intermediate III-1 with a purity of 99.0% and a yield of 78.1%. Elemental analysis structure (molecular formula C 40 h 27 NO 3 ): theoretical value C, 84.34; H, 4.78; N, 2.46; O, 8.43; test value: C, 84.32; H, 4.77; N, 2.47; ESI-MS(m / z)(M + ): The theoretical value is 569.20, and the measured value is 569.44.
[0105] (2) In a 100ml three-necked flask, under the protection of nitrogen, add 0.03mol interme...
Embodiment 3
[0107] Embodiment 3: the synthesis of compound 12:
[0108]
[0109] (1) In a 250ml three-neck flask, under the protection of nitrogen, add 0.05mol of raw material I-1, 0.075mol of intermediate II-2, dissolve with a mixed solvent (90ml of toluene, 45ml of ethanol), and then add 0.15mol of Na 2 CO 3 aqueous solution (2M), stirred under nitrogen for 1 hour, then added 0.0005mol Pd(PPh 3 ) 4 , heating to reflux for 15 hours, sampling point plate, the reaction is complete. Naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain intermediate III-2 with a purity of 99.6% and a yield of 77.4%. Elemental analysis structure (molecular formula C 40h 27 NO 2 S): Theoretical value C, 82.03; H, 4.65; N, 2.39; O, 5.46; S, 5.47; Test value: C, 82.05; ESI-MS(m / z)(M + ): The theoretical value is 585.18, and the measured value is 585.38.
[0110] (2) In a 100ml three-neck flask, under the protection of nitrogen, add 0.03mol in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com