A carbonyl reductase mutant with improved thermostability
A mutant and reductase technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of poor thermal stability of carbonyl reductase ChKRED12, and achieve the effects of flexibility, improved thermal stability, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
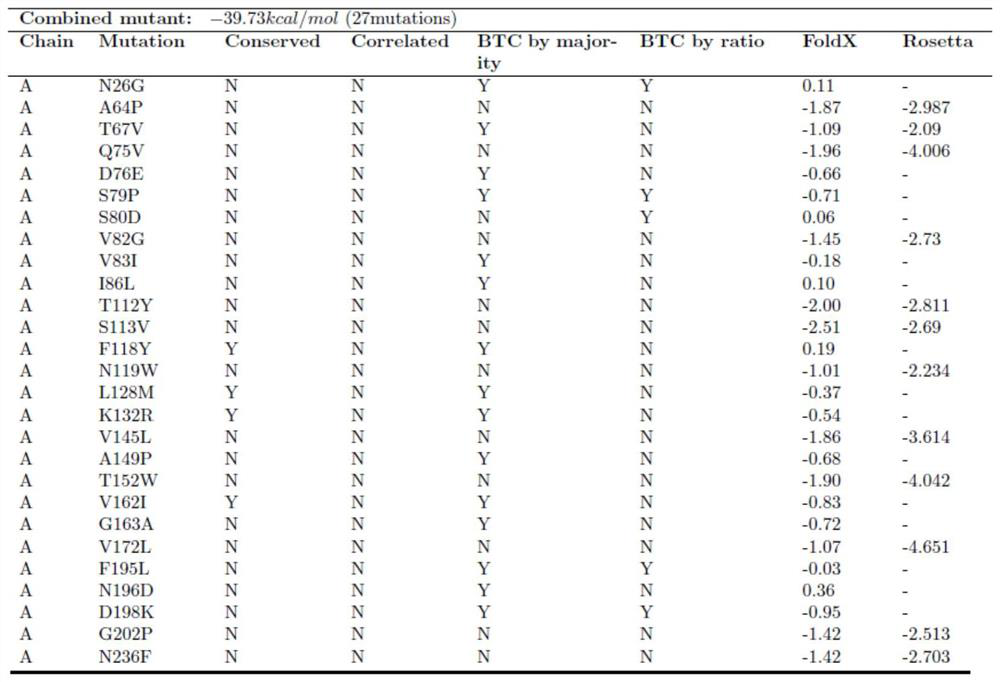
[0036] The results of the rational design method for predicting potential thermotolerance sites are shown in Figure 1 of the description. single point mutant S79P,
[0041] V162I-F: 5' - GGCGCGCTGATTGGGGCGACC - 3'
Embodiment 2
[0050] The crude enzyme activity assay reaction conditions is shown in Table 1, and the coenzyme cycle is carried out with the glucose dehydrogenase GDH. The reaction system was at 40
[0053]
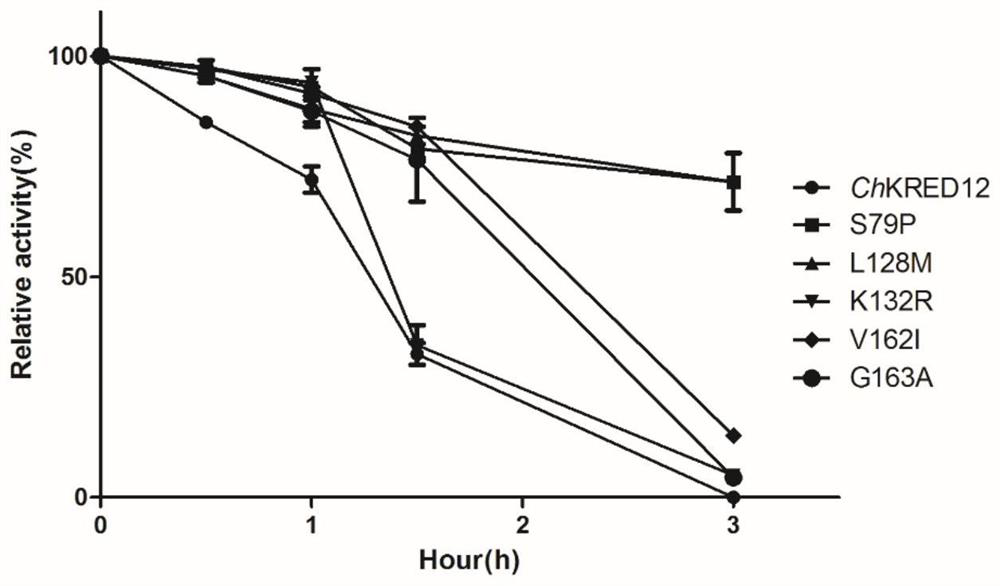
[0056] This round of mutation obtained 4 mutants S79P, L128M, V162I and G163A. Transduction of substrates by wild-type and mutants
[0058]
Embodiment 3
L128M-R: 5'-GTTACGCAGCATTTTCTGGATAAA G-3'
[0065] V162I-F: 5' - GGCGCGCTGATTGGGGCGACC - 3'
V162I-R: 5'-GGTCGCCCCAATCAGCGCGCC-3'
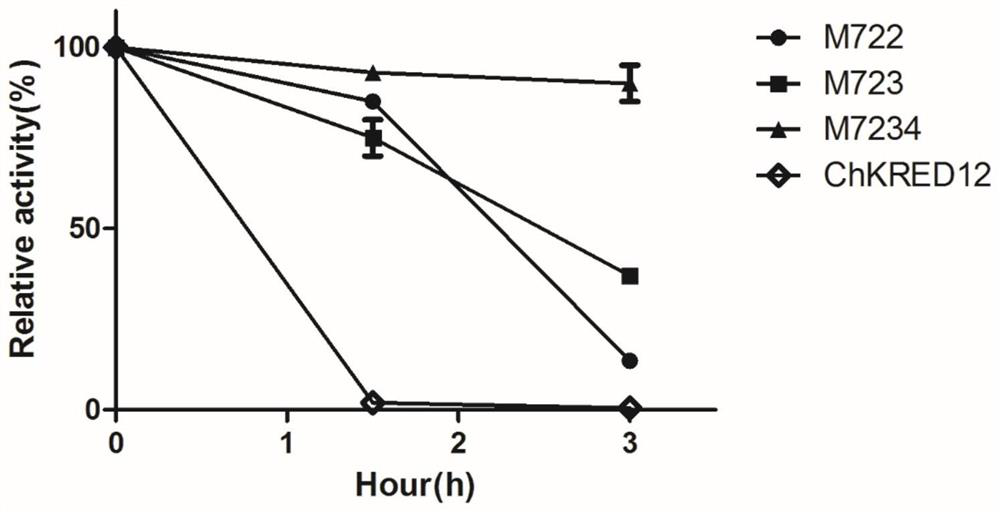
[0067] PCR conditions and operations are the same as in Example 1 to obtain a new mutant M722.
3.2 Construction of mutant M723
The site-directed mutagenesis method mutated the leucine at position 128 of mutant S79P to methionine (DNA sequence from TTA
to ATG), the glycine at position 163 was mutated to alanine (the DNA sequence was changed from GGG to GCA), and the mutant M723 was constructed.
The primers used are as follows:
L128M-F: 5'-CTTTATCCAGAAAATGCTGCGTAA C-3'
L128M-R: 5'-GTTACGCAGCATTTTCTGGATAAA G-3'
G163A-F: 5'-GGCGCGCTGGTGGCAGCGACCAAAGC-3'
G163A-R: 5' - GCTTTGGTCGCTGCCACCAGCGCGCC - 3'
[0074] PCR conditions and operations are the same as in Example 1 to obtain a new mutant M723.
3.3 Construction of mutant M7234
The site-directed mutagenesis method mutated the leucine at position 128 of mutant S79P to methionine (DNA sequ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com