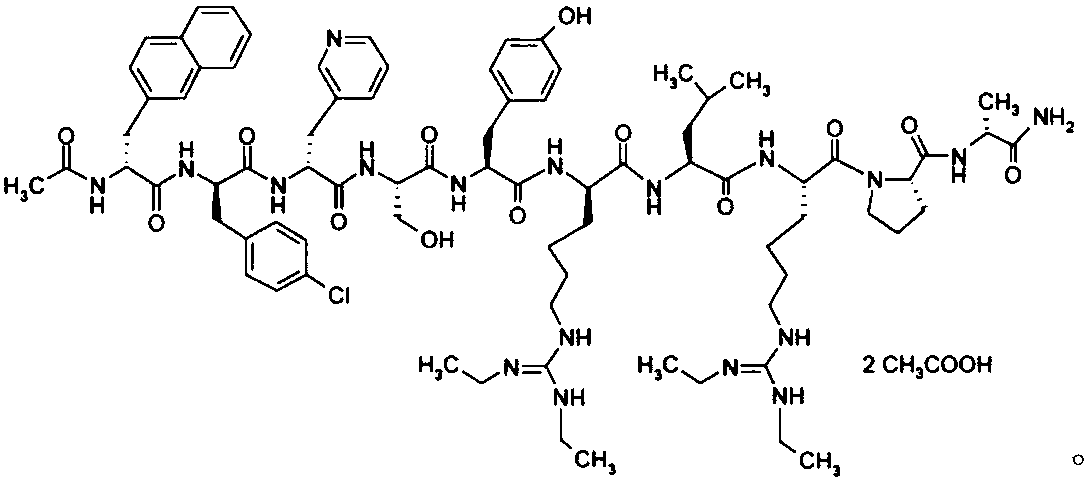
Preparation method of ganirelix acetate
A technology of ganirelix and acetic acid, applied in the field of drug synthesis, can solve problems such as toxicity, increase in processing procedures, and increase in inspection requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation of embodiment 1 ganirelix acetate solution
[0073] Mobile phase A: 2% (V / V) acetic acid in water.
[0074] Mobile phase B: 2% (V / V) acetic acid in acetonitrile.
[0075] Chromatographic column: built-in reverse-phase octadecylsilane bonded silica gel as filler.
[0076] Column equilibration: set the flow rate at 2.0L / min, select mobile phase A: mobile phase B=95:5 (V / V) and equilibrate for 50 minutes. After the equilibration is completed, set aside.
[0077] Add 400mL of purified water and 400mL of glacial acetic acid into the beaker, stir evenly, then add 125g of solid Ganirelix crude peptide (obtained by trifluoroacetic acid cracking of Ganirelix resin, HPLC purity 92.95%), after ultrasonic dissolution, Filter through a 0.45 μm filter membrane, collect the filtrate, and set the flow rate at 200 mL / min to load the sample after the column is equilibrated.
[0078] After loading the sample, set the detection wavelength to 280nm and the flow rate to 2....
Embodiment 2
[0081] The preparation of embodiment 2 ganirelix acetate refined products
[0082] Mobile phase A: 1.5% (V / V) acetic acid in water.
[0083] Mobile phase B: 1.5% (V / V) acetic acid in acetonitrile.
[0084] Chromatographic column: built-in reverse-phase octadecylsilane bonded silica gel as filler.
[0085] Column equilibration: set the flow rate at 2.0L / min, select mobile phase A:mobile phase B=85:15 (V / V) and equilibrate for 50 minutes. After the equilibration is completed, set aside.
[0086] After the column was equilibrated, the flow rate was set at 500 mL / min, and the ganirelix acetate solution prepared in Example 1 was loaded.
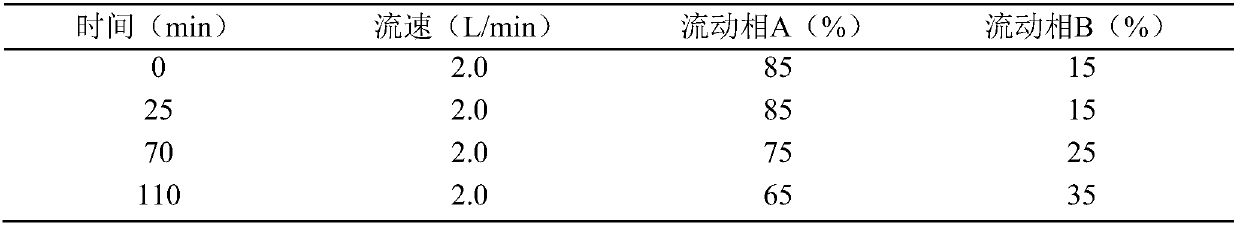
[0087] After loading the sample, set the detection wavelength to 280nm, and set the elution program according to the parameters in the table below:
[0088]
[0089]Monitor and collect the eluate of the target peak fraction, transfer the eluate to a rotary evaporator, control the temperature of the water bath at 37.5±2.5°C, and vacuum at -0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com